In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organs that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation. They are located on the left and right in the retroperitoneal space, and in adult humans are about 12 centimetres in length. They receive blood from the paired renal arteries; blood exits into the paired renal veins. Each kidney is attached to a ureter, a tube that carries excreted urine to the bladder.

The human urinary system, also known as the urinary tract or renal system, consists of the kidneys, ureters, bladder, and the urethra. The purpose of the urinary system is to eliminate waste from the body, regulate blood volume and blood pressure, control levels of electrolytes and metabolites, and regulate blood pH. The urinary tract is the body's drainage system for the eventual removal of urine. The kidneys have an extensive blood supply via the renal arteries which leave the kidneys via the renal vein. Each kidney consists of functional units called nephrons. Following filtration of blood and further processing, wastes exit the kidney via the ureters, tubes made of smooth muscle fibres that propel urine towards the urinary bladder, where it is stored and subsequently expelled through the urethra during urination. The female and male urinary system are very similar, differing only in the length of the urethra.
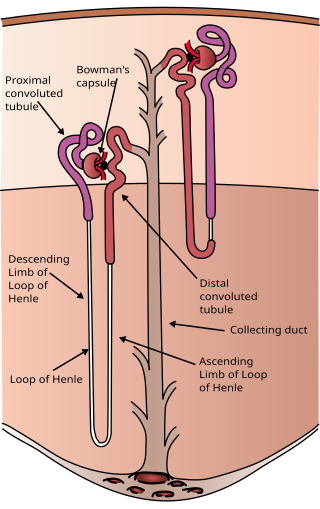
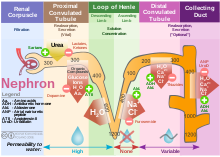
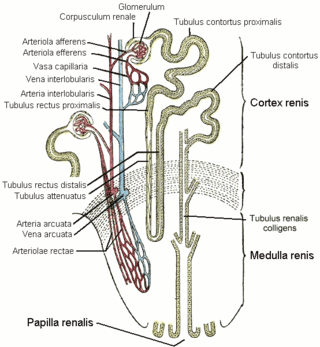
The nephron is the minute or microscopic structural and functional unit of the kidney. It is composed of a renal corpuscle and a renal tubule. The renal corpuscle consists of a tuft of capillaries called a glomerulus and a cup-shaped structure called Bowman's capsule. The renal tubule extends from the capsule. The capsule and tubule are connected and are composed of epithelial cells with a lumen. A healthy adult has 1 to 1.5 million nephrons in each kidney. Blood is filtered as it passes through three layers: the endothelial cells of the capillary wall, its basement membrane, and between the podocyte foot processes of the lining of the capsule. The tubule has adjacent peritubular capillaries that run between the descending and ascending portions of the tubule. As the fluid from the capsule flows down into the tubule, it is processed by the epithelial cells lining the tubule: water is reabsorbed and substances are exchanged ; first with the interstitial fluid outside the tubules, and then into the plasma in the adjacent peritubular capillaries through the endothelial cells lining that capillary. This process regulates the volume of body fluid as well as levels of many body substances. At the end of the tubule, the remaining fluid—urine—exits: it is composed of water, metabolic waste, and toxins.

Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.

The renin-angiotensin system (RAS), or renin-angiotensin-aldosterone system (RAAS), is a hormone system that regulates blood pressure, fluid, and electrolyte balance, and systemic vascular resistance.

Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.
The distal convoluted tubule (DCT) is a portion of kidney nephron between the loop of Henle and the collecting tubule.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.

In the kidney, the loop of Henle is the portion of a nephron that leads from the proximal convoluted tubule to the distal convoluted tubule. Named after its discoverer, the German anatomist Friedrich Gustav Jakob Henle, the loop of Henle's main function is to create a concentration gradient in the medulla of the kidney.
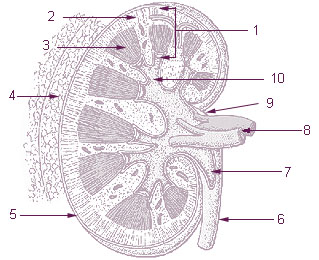
The renal medulla is the innermost part of the kidney. The renal medulla is split up into a number of sections, known as the renal pyramids. Blood enters into the kidney via the renal artery, which then splits up to form the segmental arteries which then branch to form interlobar arteries. The interlobar arteries each in turn branch into arcuate arteries, which in turn branch to form interlobular arteries, and these finally reach the glomeruli. At the glomerulus the blood reaches a highly disfavourable pressure gradient and a large exchange surface area, which forces the serum portion of the blood out of the vessel and into the renal tubules. Flow continues through the renal tubules, including the proximal tubule, the loop of Henle, through the distal tubule and finally leaves the kidney by means of the collecting duct, leading to the renal pelvis, the dilated portion of the ureter.
The syndrome of inappropriate antidiuretic hormone secretion (SIADH), also known as the syndrome of inappropriate antidiuresis (SIAD), is characterized by a physiologically inappropriate release of antidiuretic hormone (ADH) either from the posterior pituitary gland, or an abnormal non-pituitary source. Unsuppressed ADH causes a physiologically inappropriate increase in solute-free water being reabsorbed by the tubules of the kidney to the venous circulation leading to hypotonic hyponatremia.

The vasa recta of the kidney, are the straight arterioles, and the straight venules of the kidney, – a series of blood vessels in the blood supply of the kidney that enter the medulla as the straight arterioles, and leave the medulla to ascend to the cortex as the straight venules.. They lie parallel to the loop of Henle.

Bartter syndrome (BS) is a rare inherited disease characterised by a defect in the thick ascending limb of the loop of Henle, which results in low potassium levels (hypokalemia), increased blood pH (alkalosis), and normal to low blood pressure. There are two types of Bartter syndrome: neonatal and classic. A closely associated disorder, Gitelman syndrome, is milder than both subtypes of Bartter syndrome.

In renal physiology, reabsorption, more specifically tubular reabsorption, is the process by which the nephron removes water and solutes from the tubular fluid (pre-urine) and returns them to the circulating blood. It is called reabsorption (and not absorption) because these substances have already been absorbed once (particularly in the intestines) and the body is reclaiming them from a postglomerular fluid stream that is on its way to becoming urine (that is, they will soon be lost to the urine unless they are reabsorbed from the tubule into the peritubular capillaries. This happens as a result of sodium transport from the lumen into the blood by the Na+/K+ATPase in the basolateral membrane of the epithelial cells. Thus, the glomerular filtrate becomes more concentrated, which is one of the steps in forming urine. Nephrons are divided into five segments, with different segments responsible for reabsorbing different substances. Reabsorption allows many useful solutes (primarily glucose and amino acids), salts and water that have passed through Bowman's capsule, to return to the circulation. These solutes are reabsorbed isotonically, in that the osmotic potential of the fluid leaving the proximal convoluted tubule is the same as that of the initial glomerular filtrate. However, glucose, amino acids, inorganic phosphate, and some other solutes are reabsorbed via secondary active transport through cotransport channels driven by the sodium gradient.

In the renal system, peritubular capillaries are tiny blood vessels, supplied by the efferent arteriole, that travel alongside nephrons allowing reabsorption and secretion between blood and the inner lumen of the nephron. Peritubular capillaries surround the cortical parts of the proximal and distal tubules, while the vasa recta go into the medulla to approach the loop of Henle.
In the physiology of the kidney, tubuloglomerular feedback (TGF) is a feedback system inside the kidneys. Within each nephron, information from the renal tubules is signaled to the glomerulus. Tubuloglomerular feedback is one of several mechanisms the kidney uses to regulate glomerular filtration rate (GFR). It involves the concept of purinergic signaling, in which an increased distal tubular sodium chloride concentration causes a basolateral release of adenosine from the macula densa cells. This initiates a cascade of events that ultimately brings GFR to an appropriate level.

Within the nephron of the kidney, the ascending limb of the loop of Henle is a segment of the heterogenous loop of Henle downstream of the descending limb, after the sharp bend of the loop. This part of the renal tubule is divided into a thin and thick ascending limb; the thick portion is also known as the distal straight tubule, in contrast with the distal convoluted tubule downstream.
In renal physiology, renal sodium reabsorption refers to the process by which the kidneys, having filtered out waste products from the blood to be excreted as urine, re-absorb sodium ions (Na+) from the waste. It uses Na-H antiport, Na-glucose symport, sodium ion channels (minor). It is stimulated by angiotensin II and aldosterone, and inhibited by atrial natriuretic peptide.
The rock dove, Columbia livia, has a number of special adaptations for regulating water uptake and loss.

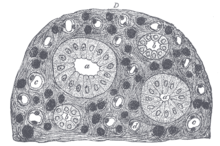
The mammalian kidneys are a pair of excretory organs of the urinary system of mammals, being functioning kidneys in postnatal-to-adult individuals. The kidneys in mammals are usually bean-shaped or externally lobulated. They are located behind the peritoneum (retroperitoneally) on the back (dorsal) wall of the body. The typical mammalian kidney consists of a renal capsule, a peripheral cortex, an internal medulla, one or more renal calyces, and a renal pelvis. Although the calyces or renal pelvis may be absent in some species. The medulla is made up of one or more renal pyramids, forming papillae with their innermost parts. Generally, urine produced by the cortex and medulla drains from the papillae into the calyces, and then into the renal pelvis, from which urine exits the kidney through the ureter. Nitrogen-containing waste products are excreted by the kidneys in mammals mainly in the form of urea.