The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.

The adrenal cortex is the outer region and also the largest part of the adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is also a secondary site of androgen synthesis.

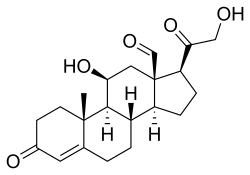
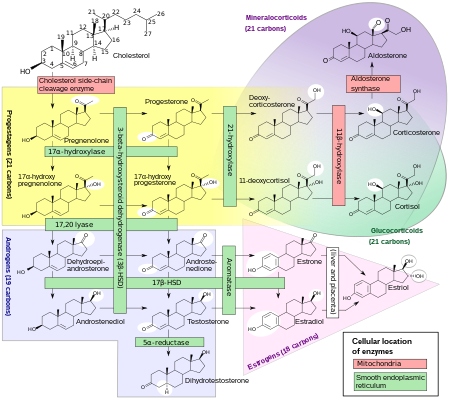
Cortisol is a steroid hormone in the glucocorticoid class of hormones and a stress hormone. When used as medication, it is known as hydrocortisone.
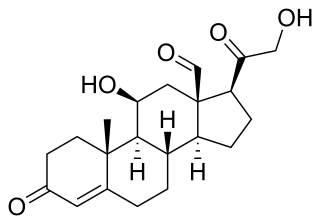
Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.
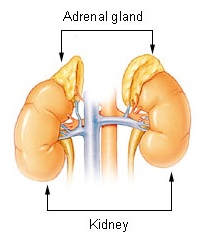
Adrenal insufficiency is a condition in which the adrenal glands do not produce adequate amounts of steroid hormones. The adrenal glands—also referred to as the adrenal cortex—normally secrete glucocorticoids, mineralocorticoids, and androgens. These hormones are important in regulating blood pressure, electrolytes, and metabolism as a whole. Deficiency of these hormones leads to symptoms ranging from abdominal pain, vomiting, muscle weakness and fatigue, low blood pressure, depression, mood and personality changes to organ failure and shock. Adrenal crisis may occur if a person having adrenal insufficiency experiences stresses, such as an accident, injury, surgery, or severe infection; this is a life-threatening medical condition resulting from severe deficiency of cortisol in the body. Death may quickly follow.
Corticotropic cells, are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.

Lipoid congenital adrenal hyperplasia is an endocrine disorder that is an uncommon and potentially lethal form of congenital adrenal hyperplasia (CAH). It arises from defects in the earliest stages of steroid hormone synthesis: the transport of cholesterol into the mitochondria and the conversion of cholesterol to pregnenolone—the first step in the synthesis of all steroid hormones. Lipoid CAH causes mineralocorticoid deficiency in affected infants and children. Male infants are severely undervirilized causing their external genitalia to look feminine. The adrenals are large and filled with lipid globules derived from cholesterol.
Congenital adrenal hyperplasia due to 17α-hydroxylase deficiency is an uncommon form of congenital adrenal hyperplasia (CAH) resulting from a mutation in the gene CYP17A1, which produces the enzyme 17α-hydroxylase. It causes decreased synthesis of cortisol and sex hormones, with resulting increase in mineralocorticoid production. Thus, common symptoms include mild cortisol deficiency, ambiguous genitalia in men or amenorrhea at puberty in women, and hypokalemic hypertension. However, partial (incomplete) deficiency often has inconsistent symptoms between patients, and affected women may be asymptomatic except for infertility.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.
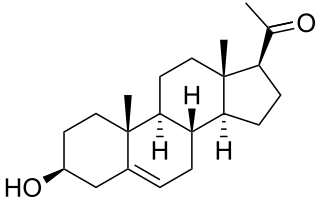
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids. In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.

The zona glomerulosa of the adrenal gland is the most superficial layer of the adrenal cortex, lying directly beneath the renal capsule. Its cells are ovoid and arranged in clusters or arches.
A mineralocorticoid receptor antagonist or aldosterone antagonist, is a diuretic drug which antagonizes the action of aldosterone at mineralocorticoid receptors. This group of drugs is often used as adjunctive therapy, in combination with other drugs, for the management of chronic heart failure. Spironolactone, the first member of the class, is also used in the management of hyperaldosteronism and female hirsutism. Most antimineralocorticoids, including spironolactone, are steroidal spirolactones. Finerenone is a nonsteroidal antimineralocorticoid.
In humans and other animals, the adrenocortical hormones are hormones produced by the adrenal cortex, the outer region of the adrenal gland. These polycyclic steroid hormones have a variety of roles that are crucial for the body's response to stress, and they also regulate other functions in the body. Threats to homeostasis, such as injury, chemical imbalances, infection, or psychological stress, can initiate a stress response. Examples of adrenocortical hormones that are involved in the stress response are aldosterone and cortisol. These hormones also function in regulating the conservation of water by the kidneys and glucose metabolism, respectively.
Pseudohyperaldosteronism is a medical condition which mimics the effects of elevated aldosterone (hyperaldosteronism) by presenting with high blood pressure, low blood potassium levels (hypokalemia), metabolic alkalosis, and low levels of plasma renin activity (PRA). However, unlike hyperaldosteronism, this conditions exhibits low or normal levels of aldosterone in the blood. Causes include genetic disorders, acquired conditions, metabolic disorders, and dietary imbalances including excessive consumption of licorice. Confirmatory diagnosis depends on the specific cause and may involve blood tests, urine tests, or genetic testing; however, all forms of this condition exhibit abnormally low concentrations of both plasma renin activity (PRA) and plasma aldosterone concentration (PAC) which differentiates this group of conditions from other forms of secondary hypertension. Treatment is tailored to the specific cause and focuses on symptom control, blood pressure management, and avoidance of triggers.

11-Deoxycorticosterone (DOC), or simply deoxycorticosterone, also known as 21-hydroxyprogesterone, as well as desoxycortone (INN), deoxycortone, and cortexone, is a steroid hormone produced by the adrenal gland that possesses mineralocorticoid activity and acts as a precursor to aldosterone. It is an active (Na+-retaining) mineralocorticoid. As its names indicate, 11-deoxycorticosterone can be understood as the 21-hydroxy-variant of progesterone or as the 11-deoxy-variant of corticosterone.

The mineralocorticoid receptor, also known as the aldosterone receptor or nuclear receptor subfamily 3, group C, member 2, (NR3C2) is a protein that in humans is encoded by the NR3C2 gene that is located on chromosome 4q31.1-31.2.
3β-Hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD) is an enzyme that catalyzes the biosynthesis of the steroid progesterone from pregnenolone, 17α-hydroxyprogesterone from 17α-hydroxypregnenolone, and androstenedione from dehydroepiandrosterone (DHEA) in the adrenal gland. It is the only enzyme in the adrenal pathway of corticosteroid synthesis that is not a member of the cytochrome P450 family. It is also present in other steroid-producing tissues, including the ovary, testis and placenta. In humans, there are two 3β-HSD isozymes encoded by the HSD3B1 and HSD3B2 genes.
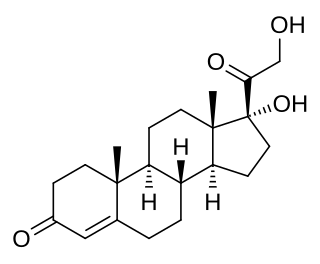
11-Deoxycortisol, also known as cortodoxone (INN), cortexolone as well as 17α,21-dihydroxyprogesterone or 17α,21-dihydroxypregn-4-ene-3,20-dione, is an endogenous glucocorticoid steroid hormone, and a metabolic intermediate toward cortisol. It was first described by Tadeusz Reichstein in 1938 as Substance S, thus has also been referred to as Reichstein's Substance S or Compound S.
Glucocorticoid remediable aldosteronism also describable as aldosterone synthase hyperactivity, is an autosomal dominant disorder in which the increase in aldosterone secretion produced by ACTH is no longer transient.

The corticosteroid receptors are receptors for corticosteroids. Corticosteroid receptors mediate the target organ response to the major products of the adrenal cortex, glucocorticoids, and mineralocorticoids. They are members of the intracellular receptor superfamily which is highly evolutionarily conserved, and includes receptors for thyroid hormones, vitamin D, sex steroids, and retinoids. They include the following two nuclear receptors: