 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɛpˈlɛrənoʊn/ |
| Trade names | Inspra, others |
| Other names | SC-66110; CGP-30083; 9-11α-Epoxymexrenone; 9,11α-Epoxy-7α-methoxycarbonyl-3-oxo-17α-pregn-4-ene-21,17-carbolactone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603004 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~70% [1] |
| Protein binding | ~50% (33–60%) (primarily to α1-acid glycoprotein) [1] [2] |
| Metabolism | Liver (CYP3A4) [1] [2] |
| Metabolites | 6β-OH-EPL, 6β,21-OH-EPL, 21-OH-EPL, 3α,6β-OH-EPL [1] (All inactive) [1] |
| Elimination half-life | 4–6 hours [3] |
| Excretion | Urine (67%), feces (32%) [4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.106.615 |
| Chemical and physical data | |
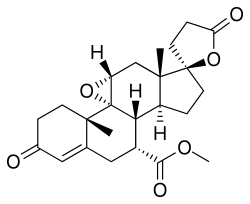
| Formula | C24H30O6 |
| Molar mass | 414.498 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Eplerenone, marketed under brand name Inspa or Espler, is an aldosterone antagonist used primarily in the treatment of heart failure with reduced ejection fraction (HFrEF), particularly following myocardial infarction. [5] [6] It may also be considered as an add-on therapy in resistant hypertension; [7] however, the majority of evidence in this setting supports the use of spironolactone (another drug in a same class), with fewer studies directly evaluating eplerenone. [8]
Contents
- Medical uses
- Heart failure
- Hypertension
- Central serous chorioretinopathy
- Adverse effects
- Interactions
- Pharmacology
- Society and culture
- Economics
- Brand names
- References
It is also a steroidal antimineralocorticoid of the spirolactone group and a selective aldosterone receptor antagonist (SARA). [9]