 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Invirase, Fortovase |
| Other names | SQV |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696001 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~4% (without ritonavir boosting) [3] |
| Protein binding | 98% |
| Metabolism | Liver, mainly by CYP3A4 |
| Elimination half-life | 9–15 hours |
| Excretion | feces (81%) and urine (3%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
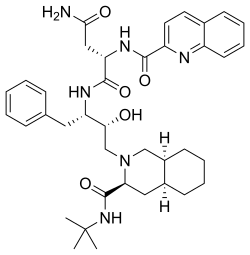
| Formula | C38H50N6O5 |
| Molar mass | 670.855 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Saquinavir, sold under the brand name Invirase among others, is an antiretroviral medication used together with other medications to treat or prevent HIV/AIDS. [4] Typically it is used with ritonavir or lopinavir/ritonavir to increase its effect. [4] It is taken by mouth. [4]
Contents
- Medical uses
- Side effects
- Bioavailability and drug interactions
- Mechanism of action
- History
- Society and culture
- Economics
- Formulations
- References
- External links
Common side effects include nausea, vomiting, diarrhea, and feeling tired. [4] More serious side effects include problems with QT prolongation, heart block, high blood lipids, and liver problems. [4] It appears to be safe in pregnancy. [4] It is in the protease inhibitor class and works by blocking the HIV protease. [4]
Saquinavir was patented in 1988 and first sold in 1995. [5] [6]
