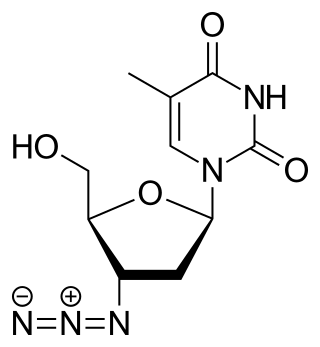
Zidovudine (ZDV), also known as azidothymidine (AZT), is an antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use in combination with other antiretrovirals. It may be used to prevent mother-to-child spread during birth or after a needlestick injury or other potential exposure. It is sold both by itself and together as lamivudine/zidovudine and abacavir/lamivudine/zidovudine. It can be used by mouth or by slow injection into a vein.
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.

Lamivudine, commonly called 3TC, is an antiretroviral medication used to prevent and treat HIV/AIDS. It is also used to treat chronic hepatitis B when other options are not possible. It is effective against both HIV-1 and HIV-2. It is typically used in combination with other antiretrovirals such as zidovudine and abacavir. Lamivudine may be included as part of post-exposure prevention in those who have been potentially exposed to HIV. Lamivudine is taken by mouth as a liquid or tablet.

Entecavir (ETV), sold under the brand name Baraclude, is an antiviral medication used in the treatment of hepatitis B virus (HBV) infection. In those with both HIV/AIDS and HBV antiretroviral medication should also be used. Entecavir is taken by mouth as a tablet or solution.

Tibotec was a pharmaceutical company with a focus on research and development for the treatment of infectious diseases such as HIV/AIDS and hepatitis C. The company was founded in 1994 and then acquired by Johnson & Johnson and merged into its Janssen Pharmaceuticals division in 2002.

Paul Adriaan Jan, Baron Janssen was a Belgian physician. He was the founder of Janssen Pharmaceutica, a pharmaceutical company with over 20,000 employees which is now a subsidiary of Johnson & Johnson.

The Rega Institute for Medical Research is a Belgian scientific establishment that is part of the Catholic University of Leuven (Leuven) in central Belgium. The Rega Institute is an interfacultary biomedical research institute of the Catholic University of Leuven and consists of departments of medicine and pharmacology.

Rudi Pauwels is a Belgian pharmacologist and biotech entrepreneur.

Loviride is an experimental antiviral drug manufactured by Janssen that is active against HIV. Loviride is a non-nucleoside reverse transcriptase inhibitor (NNRTI) that entered phase III clinical trials in the late 1990s, but failed to gain marketing approval because of poor potency. It is of clinical significance only in those patients who were enrolled in clinical trials to evaluate loviride, because in those trials loviride was often given alone and with no companion drug, leading to a high probability of developing reverse transcriptase mutations such as K103N which result in cross-class resistance to the NNRTIs efavirenz and nevirapine.

A resistance mutation is a mutation in a virus gene that allows the virus to become resistant to treatment with a particular antiviral drug. The term was first used in the management of HIV, the first virus in which genome sequencing was routinely used to look for drug resistance. At the time of infection, a virus will infect and begin to replicate within a preliminary cell. As subsequent cells are infected, random mutations will occur in the viral genome. When these mutations begin to accumulate, antiviral methods will kill the wild type strain, but will not be able to kill one or many mutated forms of the original virus. At this point a resistance mutation has occurred because the new strain of virus is now resistant to the antiviral treatment that would have killed the original virus. Resistance mutations are evident and widely studied in HIV due to its high rate of mutation and prevalence in the general population. Resistance mutation is now studied in bacteriology and parasitology.

Etravirine is a drug used for the treatment of HIV. Etravirine is a non-nucleoside reverse transcriptase inhibitor (NNRTI). Unlike the currently available agents in the class, resistance to other NNRTIs does not seem to confer resistance to etravirine. Etravirine is marketed by Janssen, a subsidiary of Johnson & Johnson. In January 2008, the Food and Drug Administration approved its use for patients with established resistance to other drugs, making it the 30th anti-HIV drug approved in the United States and the first to be approved in 2008. It was also approved for use in Canada on April 1, 2008.

Rilpivirine, sold under the brand names Edurant and Rekambys, is a medication, developed by Tibotec, used for the treatment of HIV/AIDS. It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency, longer half-life and reduced side-effect profile compared with older NNRTIs such as efavirenz.
Paul J. Lewi was a Belgian scientist, who elaborated Spectral Map Analysis in 1975 and was one of the cofounders of chemometrics in 1983. Paul Lewi was married with Godelieve Debruyne and they have together 3 children and with Philomena Van Bylen, with 2 children.

Stampidine is an experimental nucleoside reverse transcriptase inhibitor (NRTI) with anti-HIV activity.
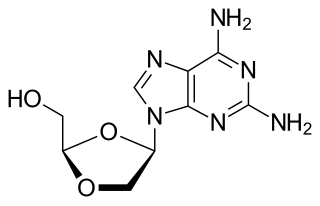
Amdoxovir is a pharmaceutical drug that has undergone research for the treatment of HIV/AIDS. It acts as a nucleoside reverse transcriptase inhibitor (NRTI). The drug was discovered by Raymond F. Schinazi and C.K. Chu and developed by RFS Pharma.

Ateviridine is a non-nucleoside reverse transcriptase inhibitor that has been studied for the treatment of HIV.
Non-nucleoside reverse-transcriptase inhibitors (NNRTIs) are antiretroviral drugs used in the treatment of human immunodeficiency virus (HIV). NNRTIs inhibit reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of HIV. RT is one of the most popular targets in the field of antiretroviral drug development.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.

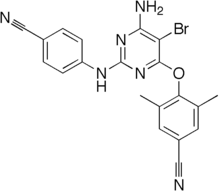
Fosdevirine is an experimental antiviral agent of the non-nucleoside reverse transcriptase inhibitor class that was studied for potential use in the treatment of HIV-AIDS.

Azvudine is an antiviral drug which acts as a reverse transcriptase inhibitor. It was discovered for the treatment of Hepatitis C and has since been investigated for use against other viral diseases such as AIDS and COVID-19, for which it was granted conditional approval in China.