Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.

Didanosine, sold under the brand name Videx, is a medication used to treat HIV/AIDS. It is used in combination with other medications as part of highly active antiretroviral therapy (HAART). It is of the reverse-transcriptase inhibitor class.

Zalcitabine, also called dideoxycytidine, is a nucleoside analog reverse-transcriptase inhibitor (NRTI) sold under the trade name Hivid. Zalcitabine was the third antiretroviral to be approved by the Food and Drug Administration (FDA) for the treatment of HIV/AIDS. It is used as part of a combination regimen.

Lamivudine, commonly called 3TC, is an antiretroviral medication used to prevent and treat HIV/AIDS. It is also used to treat chronic hepatitis B when other options are not possible. It is effective against both HIV-1 and HIV-2. It is typically used in combination with other antiretrovirals such as zidovudine, dolutegravir, and abacavir. Lamivudine may be included as part of post-exposure prevention in those who have been potentially exposed to HIV. Lamivudine is taken by mouth as a liquid or tablet.

Nevirapine (NVP), sold under the brand name Viramune among others, is a medication used to treat and prevent HIV/AIDS, specifically HIV-1. It is generally recommended for use with other antiretroviral medications. It may be used to prevent mother to child spread during birth but is not recommended following other exposures. It is taken by mouth.

Entecavir (ETV), sold under the brand name Baraclude, is an antiviral medication used in the treatment of hepatitis B virus (HBV) infection. In those with both HIV/AIDS and HBV antiretroviral medication should also be used. Entecavir is taken by mouth as a tablet or solution.
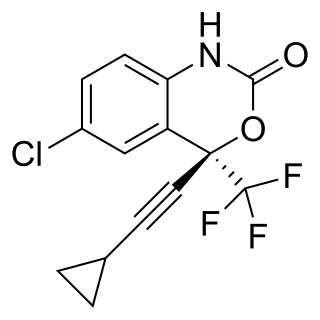
Efavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is sold both by itself and in combination as efavirenz/emtricitabine/tenofovir. It is taken by mouth.

Lamivudine/zidovudine, sold under the brand name Combivir among others, is a fixed-dose combination antiretroviral medication used to treat HIV/AIDS. It contains two antiretroviral medications, lamivudine and zidovudine. It is used together with other antiretrovirals. It is taken by mouth twice a day.

Delavirdine (DLV) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) marketed by ViiV Healthcare. It is used as part of highly active antiretroviral therapy (HAART) for the treatment of human immunodeficiency virus (HIV) type 1. It is presented as the mesylate. The recommended dosage is 400 mg, three times a day.

Efavirenz/emtricitabine/tenofovir, sold under the brand name Atripla among others, is a fixed-dose combination antiretroviral medication used to treat HIV/AIDS. It contains efavirenz, emtricitabine, and tenofovir disoproxil. It can be used by itself or together with other antiretroviral medications. It is taken by mouth.

A resistance mutation is a mutation in a virus gene that allows the virus to become resistant to treatment with a particular antiviral drug. The term was first used in the management of HIV, the first virus in which genome sequencing was routinely used to look for drug resistance. At the time of infection, a virus will infect and begin to replicate within a preliminary cell. As subsequent cells are infected, random mutations will occur in the viral genome. When these mutations begin to accumulate, antiviral methods will kill the wild type strain, but will not be able to kill one or many mutated forms of the original virus. At this point a resistance mutation has occurred because the new strain of virus is now resistant to the antiviral treatment that would have killed the original virus. Resistance mutations are evident and widely studied in HIV due to its high rate of mutation and prevalence in the general population. Resistance mutation is now studied in bacteriology and parasitology.

Diarylpyrimidines (DAPY) and diaryltriazines (DATA) are two closely related classes of molecules resembling the pyrimidine nucleotides found in DNA. They show great potency in inhibiting the activity of HIV reverse transcriptase. Several compounds in this class are non-nucleoside reverse transcriptase inhibitors used clinically in the treatment of HIV/AIDS, notably etravirine and rilpivirine.

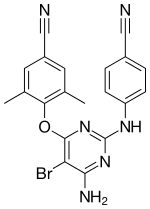
Rilpivirine, sold under the brand names Edurant and Rekambys, is a medication, developed by Tibotec, used for the treatment of HIV/AIDS. It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency, longer half-life and reduced side-effect profile compared with older NNRTIs such as efavirenz.

Stampidine is an experimental nucleoside reverse transcriptase inhibitor (NRTI) with anti-HIV activity.
Non-nucleoside reverse-transcriptase inhibitors (NNRTIs) are antiretroviral drugs used in the treatment of human immunodeficiency virus (HIV). NNRTIs inhibit reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of HIV. RT is one of the most popular targets in the field of antiretroviral drug development.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.

Bictegravir/emtricitabine/tenofovir alafenamide, sold under the brand name Biktarvy, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. One tablet, taken orally once daily, contains 50 mg bictegravir, 200 mg emtricitabine, and 25 mg tenofovir alafenamide. It was approved for use in the United States in February 2018, and for use in the European Union in June 2018.
Dolutegravir/rilpivirine, sold under the brand name Juluca, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It contains the medicines dolutegravir and rilpivirine. It is taken by mouth.

Cabotegravir/rilpivirine, sold under the brand name Cabenuva, is a co-packaged antiretroviral medication for the treatment of HIV/AIDS. It contains cabotegravir and rilpivirine in a package with two separate injection vials.
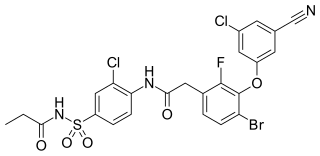
Elsulfavirine is drug used to treat HIV infection. It is a non-nucleoside reverse transcriptase inhibitor (NNRTI). Elsulfavirine is a prodrug which is metabolized to the active antiviral agent deselsulfavirine. It was developed by the Russian company Viriom.