Related Research Articles
Antiviral drugs are a class of medication used specifically for treating viral infections rather than bacterial ones. Most antivirals are used for specific viral infections, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit their development.

An HIV vaccine may have the purpose of protecting individuals who do not have HIV from being infected with the virus, or treating an HIV-infected person. There are two approaches to an HIV vaccine: an active vaccination approach in which a vaccine aims to induce an immune response against HIV; and a passive vaccination approach in which preformed antibodies against HIV are administered.

A lymphocyte is one of the subtypes of a white blood cell in a vertebrate's immune system. Lymphocytes include natural killer cells, T cells, and B cells. They are the main type of cell found in lymph, which prompted the name "lymphocyte".

The adaptive immune system, also known as the acquired immune system or, more rarely, as the specific immune system, is a subsystem of the overall immune system that is composed of highly specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The acquired immune system is one of the two main immunity strategies found in vertebrates.
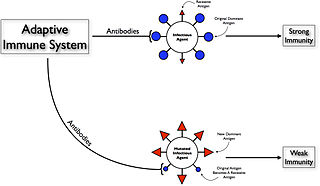
Original antigenic sin, also known as the Hoskins effect, refers to the propensity of the body's immune system to preferentially utilize immunological memory based on a previous infection when a second slightly different version of that foreign entity is encountered. This leaves the immune system "trapped" by the first response it has made to each antigen, and unable to mount potentially more effective responses during subsequent infections. The phenomenon of original antigenic sin has been described in relation to influenza virus, dengue fever, human immunodeficiency virus (HIV), and to several other viruses.

The International AIDS Vaccine Initiative is a global not-for-profit, public-private partnership working to accelerate the development of vaccines to prevent HIV infection and AIDS. IAVI researches and develops vaccine candidates, conducts policy analyses, serves as an advocate for the HIV prevention field and engages communities in the trial process and AIDS vaccine education. The organization takes a comprehensive approach to HIV and AIDS that supports existing HIV prevention and treatment programs while emphasizing the need for new AIDS prevention tools. It also works to ensure that future vaccines will be accessible to all who need them.

Envelope glycoprotein GP120 is a glycoprotein exposed on the surface of the HIV envelope. It was discovered by Professors Tun-Hou Lee and Myron "Max" Essex of the Harvard School of Public Health in 1988. The 120 in its name comes from its molecular weight of 120 kDa. Gp120 is essential for virus entry into cells as it plays a vital role in attachment to specific cell surface receptors. These receptors are DC-SIGN, Heparan Sulfate Proteoglycan and a specific interaction with the CD4 receptor, particularly on helper T-cells. Binding to CD4 induces the start of a cascade of conformational changes in gp120 and gp41 that lead to the fusion of the viral membrane with the host cell membrane. Binding to CD4 is mainly electrostatic although there are van der Waals interactions and hydrogen bonds.

The HIV Prevention Trials Network (HPTN) is a worldwide collaborative clinical trials network that brings together investigators, ethicists, community and other partners to develop and test the safety and efficacy of interventions designed to prevent the acquisition and transmission of HIV. HPTN studies evaluate new HIV prevention interventions and strategies in populations and geographical regions that bear a disproportionate burden of infection. The HPTN is committed to the highest ethical standards for its clinical trials and recognizes the importance of community engagement in all phases of the research process.
Integrase inhibitors (INIs) are a class of antiretroviral drug designed to block the action of integrase, a viral enzyme that inserts the viral genome into the DNA of the host cell. Since integration is a vital step in retroviral replication, blocking it can halt further spread of the virus. Integrase inhibitors were initially developed for the treatment of HIV infection, but have been applied to other retroviruses. The class of integrase inhibitors called integrase strand transfer inhibitors (INSTIs) are in established medical use. Other classes, such as integrase binding inhibitors (INBIs), are still experimental.
HIV superinfection is a condition in which a person with an established human immunodeficiency virus infection acquires a second strain of HIV, often of a different subtype. These can form a recombinant strain that co-exists with the strain from the initial infection, as well as the strain from the new virus, and may cause more rapid disease progression or carry multiple resistances to certain HIV medications.
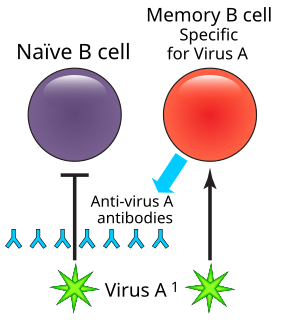
Antibody-dependent enhancement (ADE) occurs when non-neutralizing antiviral proteins facilitate virus entry into host cells, leading to increased infectivity in the cells. Some cells do not have the usual receptors on their surfaces that viruses use to gain entry. The antiviral proteins bind to antibody Fc receptors that some of these cells have in the plasma membrane. The viruses bind to the antigen binding site at the other end of the antibody. ADE is common in cells cultured in the laboratory, but rarely occurs in vivo except for dengue virus. This virus can use this mechanism to infect human macrophages, causing a normally mild viral infection to become life-threatening.
Long-term nonprogressors (LTNPs), sometimes also called elite controllers, are individuals infected with HIV, who maintain a CD4 count greater than 500 without antiretroviral therapy with a detectable viral load. Many of these patients have been HIV positive for 30 years without progressing to the point of needing to take medication in order not to develop AIDS. They have been the subject of a great deal of research, since an understanding of their ability to control HIV infection may lead to the development of immune therapies or a therapeutic vaccine. The classification "Long-term non-progressor" is not permanent, because some patients in this category have gone on to develop AIDS.
2F5 is a broadly neutralizing human monoclonal antibody (mAb) that has been shown to bind to and neutralize HIV-1 in vitro, making it a potential candidate for use in vaccine synthesis. 2F5 recognizes an epitope in the membrane-proximal external region (MPER) of HIV-1 gp41. 2F5 then binds to this epitope and its constant region interacts with the viral lipid membrane, which neutralizes the virus.
A neutralizing antibody (NAb) is an antibody that defends a cell from an antigen or infectious body by neutralizing any effect it has biologically. An example of a neutralizing antibody is diphtheria antitoxin, which can neutralize the biological effects of diphtheria toxin.
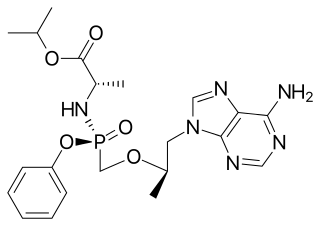
Tenofovir alafenamide is a nucleotide reverse transcriptase inhibitor and a prodrug of tenofovir. It was developed by Gilead Sciences based on the protide technology of Chris McGuigan for use in the treatment of HIV infection and chronic hepatitis B, and is applied in the form of tenofovir alafenamide fumarate (TAF). Closely related to the commonly used reverse-transcriptase inhibitor tenofovir disoproxil fumarate (TDF), TAF has greater antiviral activity and better distribution into lymphoid tissues than that agent. Vemlidy was approved by the U.S. Food and Drug Administration (FDA) in November 2016.
Broadly Neutralizing HIV-1 Antibodies (bNAbs) are neutralizing antibodies which neutralize multiple HIV-1 viral strains. bNAbs are unique in that they target conserved epitopes of the virus, meaning the virus may mutate, but the targeted epitopes will still exist. In contrast, non-bNAbs are specific for individual viral strains with unique epitopes. The discovery of bNAbs has led to an important area of research, namely, discovery of a vaccine, not only limited to HIV, but also other rapidly mutating viruses like Influenza.

Michel C. Nussenzweig is a professor and head of the Laboratory of Molecular Immunology at The Rockefeller University and a Howard Hughes Medical Institute investigator. He is a member of both the US National Academy of Medicine and the US National Academy of Sciences.
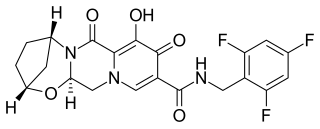
Bictegravir is a drug of the integrase inhibitor class that was copied from Dolutegravir by scientists at Gilead Sciences ; in vitro and clinical results were presented by Gilead in the summer of 2016. In 2016, bictegravir was in a Phase 3 trial as part of a single tablet regimen in combination with tenofovir alafenamide (TAF) and emtricitabine (FTC) for the treatment of HIV-1 infection and the combination drug bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy) was approved for use in the United States in 2018.
Intrastructural help (ISH) is where T and B cells cooperate to help or suppress an immune response gene. ISH has proven effective for the treatment of influenza, rabies related lyssavirus, hepatitis B, and the HIV virus. This process was used in 1979 to observe that T cells specific to the influenza virus could promote the stimulation of hemagglutinin specific B cells and elicit an effective humoral immune response. It was later applied to the lyssavirus and was shown to protect raccoons from lethal challenge. The ISH principle is especially beneficial because relatively invariable structural antigens can be used for the priming of T-cells to induce humoral immune response against variable surface antigens. Thus, the approach has also transferred well for the treatment of hepatitis B and HIV.
UB-421 is an experimental HIV antibody, under development by United Biomedical, Inc. (UBI), headquartered in Hauppauge, New York, U.S. for use in the treatment of HIV infection. By blocking the CDR2 domain of the CD4 receptor of the virus, it prevents initial viral attachment to the host T cell and entry into the host immune cell via a competitive inhibition mechanism. The antibody is unlikely to promote resistance to itself via generation of CD4-independent virus, and has performed well in phase 2 open-label trials. Additionally, it offers hope to HIV patients whose infection has become multi-drug resistant. Furthermore, the antibody has shown long term suppression, which requires the patient to be treated less often, which improves treatment adherence. Previous experimental infusions of broadly neutralizing antibodies, or BNabs, have suppressed HIV for about two weeks by targeting proteins on the virus itself, but the rapid mutation rate of HIV induces antibody-resistant strains that render the treatment ineffective. UB-421 theoretically avoids this possibility by blocking a stable human protein that HIV uses to infect T cells. Its advantages include its competitive inhibition, a high affinity of UB-421 to CD4 T cells which is 100 times stronger than HIV, its neutralization of multiple sub-types of HIV, its inhibition of both cell-to-cell and cell-free transmission of HIV, and its immune modulation of training the immune system to better attack HIV, all of which provide potential as monotherapy.
References
- ↑ "GS-9722: FIRST-IN-CLASS EFFECTOR-ENHANCED BROADLY NEUTRALIZING ANTIBODY FOR HIV CURE". CroiConference.Org.
- ↑ "Gilead Announces Data from New Preclinical Study Evaluating a Combination of an Investigational TLR7 Agonist and an Investigational HIV Envelope Targeting Antibody in SHIV-Infected, Virally Suppressed Monkeys". Gilead.com.