An international nonproprietary name (INN) is an official generic and nonproprietary name given to a pharmaceutical drug or an active ingredient. INNs are intended to make communication more precise by providing a unique standard name for each active ingredient, to avoid prescribing errors. The INN system has been coordinated by the World Health Organization (WHO) since 1953.
Lucatumumab is a human monoclonal antibody against CD40 development of which was discontinued by Novartis in 2013 after it was investigated for the treatment of various types of cancer like multiple myeloma and follicular lymphoma.

Odanacatib is an investigational treatment for osteoporosis and bone metastasis. It is an inhibitor of cathepsin K, an enzyme involved in bone resorption.

Linagliptin, sold under the brand name Tradjenta among others, is a medication used to treat type 2 diabetes in conjunction with exercise and diet. It is generally less preferred than metformin and sulfonylureas as an initial treatment. It is taken by mouth.
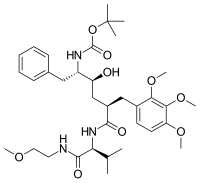
Gemopatrilat (INN) is an experimental drug that was never marketed. It acts as a vasopeptidase inhibitor. It inhibits both angiotensin-converting enzyme (ACE) and neutral endopeptidase (neprilysin).

Elinogrel (INN, USAN) was an experimental antiplatelet drug acting as a P2Y12 inhibitor. Similarly to ticagrelor and in contrast to clopidogrel, elinogrel was a reversible inhibitor that acted fast and short (for about 12 hours), and it was not a prodrug but pharmacologically active itself. The substance was used in form of its potassium salt, intravenously for acute treatment and orally for long-term treatment. Development was terminated in 2012.
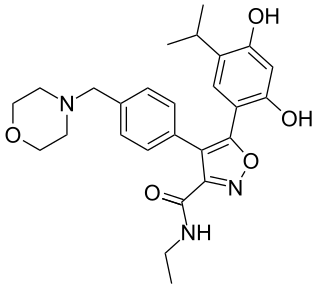
Luminespib is an experimental drug candidate for the treatment of cancer. It was discovered through a collaboration between The Institute of Cancer Research and the pharmaceutical company Vernalis and licensed to Novartis. From 2011 to 2014 it was in Phase II clinical trials. Chemically it is a resorcinylic isoxazole amide
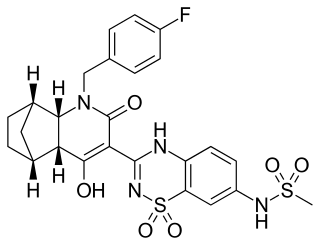
Setrobuvir was an experimental drug candidate for the treatment of hepatitis C that was discovered at Anadys Pharmaceuticals, which was acquired by Roche in 2011; Roche terminated development in July 2015. It was in Phase IIb clinical trials, used in combination with interferon and ribavirin, targeting hepatitis C patients with genotype 1.

Abediterol is a once-daily experimental drug candidate for the treatment of asthma and chronic obstructive pulmonary disease (COPD). It is currently under development by the Spanish pharmaceutical company Almirall and is in Phase II clinical trials.
Ligelizumab is a humanized IgG1 monoclonal antibody designed for the treatment of severe asthma and chronic spontaneous urticaria. It is an anti-IgE that binds to IGHE an acts as an immunomodulator.
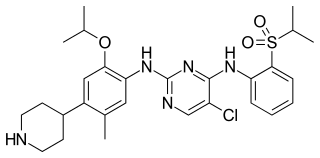
Ceritinib is a prescription-only drug used for the treatment of non-small cell lung cancer (NSCLC). It was developed by Novartis and received FDA approval for use in April 2014..Ceritinib is also sold under the brand name Spexib in few countries by Novartis.

Ipragliflozin is a pharmaceutical drug for treatment of type 2 diabetes. Ipragliflozin, jointly developed by Astellas Pharma and Kotobuki Pharmaceutical, was approved in Japan on January 17, 2014, and in Russia on May 22, 2019.
Sufotidine (INN, USAN, codenamed AH25352) is a long-acting competitive H2 receptor antagonist which was under development as an antiulcerant by Glaxo (now GlaxoSmithKline). It was planned to be a follow-up compound to ranitidine (Zantac). When taken in doses of 600 mg twice daily it induced virtually 24-hour gastric anacidity thus closely resembling the antisecretory effect of the proton pump inhibitor omeprazole. Its development was terminated in 1989 from phase III clinical trials based on the appearance of carcinoid tumors in long-term toxicity testing in rodents.

Oprozomib is an orally active second-generation proteasome inhibitor developed by Proteolix, which was acquired by Onyx Pharmaceuticals, an Amgen subsidiary, in 2009. It selectively inhibits chymotrypsin-like activity of both the constitutive proteasome (PSMB5) and immunoproteasome (LMP7).
Brolucizumab sold under trade name Beovu among others, is a humanized single-chain antibody fragment for the treatment of neovascular (wet) age-related macular degeneration (AMD).
Depatuxizumab mafodotin is an antibody-drug conjugate designed for the treatment of cancer. It is composed of an EGFR IGg1 monoclonal antibody (depatuxizumab) conjugated to the tubulin inhibitor monomethyl auristatin F via a stable maleimidocaproyl link.
Spartalizumab is a monoclonal antibody and checkpoint inhibitor that is being investigated for melanoma.
Ianalumab is a monoclonal antibody that is being investigated for autoimmune hepatitis, multiple sclerosis, pemphigus vulgaris, rheumatoid arthritis, Sjögren syndrome, and systemic lupus erythematosus.
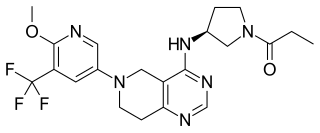
Leniolisib, sold under the brand name Joenja, is a medication used for the treatment of activated phosphoinositide 3-kinase delta syndrome (APDS). Leniolisib is a kinase inhibitor.