 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tivicay, Tivicay PD, Instgra |
| Other names | GSK572, S-349572 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613043 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a [4] |
| Protein binding | ≥98.9% |
| Metabolism | UGT1A1 and CYP3A |
| Elimination half-life | ~14 hours |
| Excretion | Feces (53%) and urine (18.9%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.237.735 |
| Chemical and physical data | |
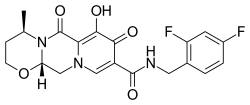
| Formula | C20H19F2N3O5 |
| Molar mass | 419.385 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dolutegravir (DTG), sold under the brand name Tivicay or Instgra, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS. [6] It may also be used, as part of post exposure prophylaxis, to prevent HIV infection following potential exposure. [7] It is taken by mouth. [6]
Contents
Common side effects include trouble sleeping, feeling tired, diarrhea, high blood sugar, and headache. [7] Severe side effects may include allergic reactions and liver problems. [7] Concerns that usage during pregnancy can result in harm to the baby have been refuted by further studies that show there is no statistical difference in neural tube defects from the usage of dolutegravir compared to other antiretrovirals. [8] It is unclear if use during breastfeeding is safe. [7] Dolutegravir is an HIV integrase strand transfer inhibitor which blocks the functioning of HIV integrase which is needed for viral replication. [7]
Dolutegravir was approved for medical use in the United States in 2013. [7] It is on the World Health Organization's List of Essential Medicines. [9] Abacavir/dolutegravir/lamivudine, a combination with abacavir and lamivudine is also available. [7] [10] [11] As of 2019, the World Health Organization (WHO) recommends DTG as the first- and second-line treatment for all persons with HIV. [12]