The genome and proteins of HIV (human immunodeficiency virus) have been the subject of extensive research since the discovery of the virus in 1983. "In the search for the causative agent, it was initially believed that the virus was a form of the Human T-cell leukemia virus (HTLV), which was known at the time to affect the human immune system and cause certain leukemias. However, researchers at the Pasteur Institute in Paris isolated a previously unknown and genetically distinct retrovirus in patients with AIDS which was later named HIV." Each virion comprises a viral envelope and associated matrix enclosing a capsid, which itself encloses two copies of the single-stranded RNA genome and several enzymes. The discovery of the virus itself occurred two years following the report of the first major cases of AIDS-associated illnesses.

Envelope glycoprotein GP120 is a glycoprotein exposed on the surface of the HIV envelope. It was discovered by Professors Tun-Hou Lee and Myron "Max" Essex of the Harvard School of Public Health in 1984. The 120 in its name comes from its molecular weight of 120 kDa. Gp120 is essential for virus entry into cells as it plays a vital role in attachment to specific cell surface receptors. These receptors are DC-SIGN, Heparan Sulfate Proteoglycan and a specific interaction with the CD4 receptor, particularly on helper T-cells. Binding to CD4 induces the start of a cascade of conformational changes in gp120 and gp41 that lead to the fusion of the viral membrane with the host cell membrane. Binding to CD4 is mainly electrostatic although there are van der Waals interactions and hydrogen bonds.
Entry inhibitors, also known as fusion inhibitors, are a class of antiviral drugs that prevent a virus from entering a cell, for example, by blocking a receptor. Entry inhibitors are used to treat conditions such as HIV and hepatitis D.
Env is a viral gene that encodes the protein forming the viral envelope. The expression of the env gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.
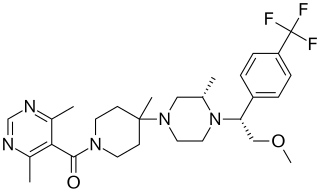
Vicriviroc, previously named SCH 417690 and SCH-D, is a pyrimidine CCR5 entry inhibitor of HIV-1. It was developed by the pharmaceutical company Schering-Plough. Merck decided to not pursue regulatory approval for use in treatment-experienced patients because the drug did not meet primary efficacy endpoints in late stage trials. Clinical trials continue in patients previously untreated for HIV.
CD4 immunoadhesin is a recombinant fusion protein consisting of a combination of CD4 and the fragment crystallizable region, similarly known as immunoglobulin. It belongs to the antibody (Ig) gene family. CD4 is a surface receptor for human immunodeficiency virus (HIV). The CD4 immunoadhesin molecular fusion allow the protein to possess key functions from each independent subunit. The CD4 specific properties include the gp120-binding and HIV-blocking capabilities. Properties specific to immunoglobulin are the long plasma half-life and Fc receptor binding. The properties of the protein means that it has potential to be used in AIDS therapy as of 2017. Specifically, CD4 immunoadhesin plays a role in antibody-dependent cell-mediated cytotoxicity (ADCC) towards HIV-infected cells. While natural anti-gp120 antibodies exhibit a response towards uninfected CD4-expressing cells that have a soluble gp120 bound to the CD4 on the cell surface, CD4 immunoadhesin, however, will not exhibit a response. One of the most relevant of these possibilities is its ability to cross the placenta.

Griffithsin is a protein isolated from the red algae Griffithsia. It has a 121-amino acid sequence which exhibits a Jacalin-like lectin fold. Several structures of this protein have been solved by X-ray crystallography and deposited in the PDB. It has been shown in vitro to be a highly potent HIV entry inhibitor. It is currently being investigated as a potential microbicide for use in the prevention of the transmission of HIV.

Neutral alpha-glucosidase C is an enzyme that in humans is encoded by the GANC gene.

Neutral alpha-glucosidase AB is an enzyme that in humans is encoded by the GANAB gene.

Interferon alpha-7 is a protein that in humans is encoded by the IFNA7 gene.

HLA class II histocompatibility antigen, DX beta chain is a protein that in humans is encoded by the HLA-DQB2 gene.

Mannosyl-oligosaccharide 1,2-alpha-mannosidase IA is an enzyme that in humans is encoded by the MAN1A1 gene.

Mannosyl-oligosaccharide glucosidase is an enzyme that in humans is encoded by the MOGS gene.

GBA2 is the gene that encodes the enzyme non-lysosomal glucosylceramidase in humans. It has glucosylceramidase activity.

Mannosyl-oligosaccharide 1,2-alpha-mannosidase IB is an enzyme that in humans is encoded by the MAN1A2 gene.
2F5 is a broadly neutralizing human monoclonal antibody (mAb) that has been shown to bind to and neutralize HIV-1 in vitro, making it a potential candidate for use in vaccine synthesis. 2F5 recognizes an epitope in the membrane-proximal external region (MPER) of HIV-1 gp41. 2F5 then binds to this epitope and its constant region interacts with the viral lipid membrane, which neutralizes the virus.
CCR5 receptor antagonists are a class of small molecules that antagonize the CCR5 receptor. The C-C motif chemokine receptor CCR5 is involved in the process by which HIV, the virus that causes AIDS, enters cells. Hence antagonists of this receptor are entry inhibitors and have potential therapeutic applications in the treatment of HIV infections.
In molecular biology, the CVNH domain is a conserved protein domain. It is found in the sugar-binding antiviral protein cyanovirin-N (CVN) as well as proteins from filamentous ascomycetes and in the fern Ceratopteris richardii.
Scytonema varium is a cultured cyanobacterium of the genus Scytonema. It is one of many anti viral protein producing algae. In a similar manner to Cyanovirin-N from Nostoc Ellipsosporum and griffithsin from the red algae Griffithsia, Scytonema varium secretes the broad-spectrum antiviral protein scytovirin which can inactivate both the HIV virus, and Ebola virus, offering hope of treatment for many diseases with viral etiology (cause). It is currently being investigated as a topical microbicide for HIV prophylaxis.
Scytovirin is a 95-amino acid antiviral protein isolated from the cyanobacteria Scytonema varium. It has been cultured in E. coli and its structure investigated in detail. Scytovirin is thought to be produced by the bacteria to protect itself from viruses that might otherwise attack it, but as it has broad-spectrum antiviral activity against a range of enveloped viruses, scytovirin has also been found to be useful against a range of major human pathogens, most notably HIV / AIDS but also including SARS coronavirus and filoviruses such as Ebola virus and Marburg virus. While some lectins such as cyanovirin and Urtica dioica agglutinin are thought likely to be too allergenic to be used internally in humans, studies so far on scytovirin and griffithsin have not shown a similar level of immunogenicity. Scytovirin and griffithsin are currently being investigated as potential microbicides for topical use.










