Related Research Articles
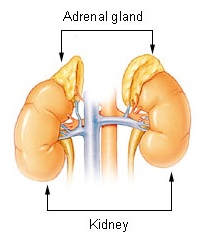
The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.

Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involved in a wide range of physiological processes, including stress response, immune response, and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels, and behavior.

The adrenal cortex is the outer region and also the largest part of an adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is also a secondary site of androgen synthesis.

A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids and sex steroids. Within those two classes are five types according to the receptors to which they bind: glucocorticoids and mineralocorticoids and androgens, estrogens, and progestogens. Vitamin D derivatives are a sixth closely related hormone system with homologous receptors. They have some of the characteristics of true steroids as receptor ligands.

Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume. When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.

Glucocorticoids are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebrate animal cell. The name "glucocorticoid" is a portmanteau and is composed from its role in regulation of glucose metabolism, synthesis in the adrenal cortex, and its steroidal structure.

Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances. The primary mineralocorticoid is aldosterone.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.
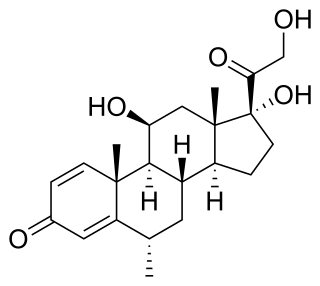
Methylprednisolone is a synthetic glucocorticoid, primarily prescribed for its anti-inflammatory and immunosuppressive effects. It is either used at low doses for chronic illnesses or used concomitantly at high doses during acute flares. Methylprednisolone and its derivatives can be administered orally or parenterally.
11β-Hydroxysteroid dehydrogenase enzymes catalyze the conversion of inert 11 keto-products (cortisone) to active cortisol, or vice versa, thus regulating the access of glucocorticoids to the steroid receptors.

Eplerenone, sold under the brand name Inspra, is an aldosterone antagonist type of potassium-sparing diuretic that is used to treat chronic heart failure and high blood pressure, particularly for patients with resistant hypertension due to elevated aldosterone. It is a steroidal antimineralocorticoid of the spirolactone group and a selective aldosterone receptor antagonist (SARA). Eplerenone is more selective than spironolactone at the mineralocorticoid receptor relative to binding at androgen, progestogen, glucocorticoid, or estrogen receptors.

Corticosteroid 11-β-dehydrogenase isozyme 2 also known as 11-β-hydroxysteroid dehydrogenase 2 is an enzyme that in humans is encoded by the HSD11B2 gene.

The mineralocorticoid receptor, also known as the aldosterone receptor or nuclear receptor subfamily 3, group C, member 2, (NR3C2) is a protein that in humans is encoded by the NR3C2 gene that is located on chromosome 4q31.1-31.2.

Loteprednol is a topical corticosteroid used to treat inflammations of the eye. It is marketed by Bausch and Lomb as Lotemax and Loterex.
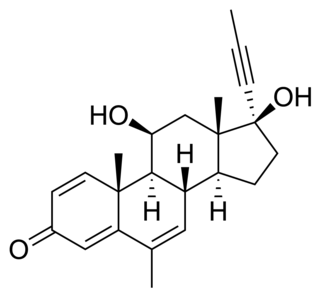
RU-28362 is a synthetic androstane glucocorticoid that was developed by Roussel Uclaf. It is a selective agonist of the glucocorticoid receptor, but not of the mineralocorticoid receptor.

The corticosteroid receptors are receptors for corticosteroids. They include the following two nuclear receptors:
Membrane steroid receptors (mSRs), also called extranuclear steroid receptors, are a class of cell surface receptors activated by endogenous steroids that mediate rapid, non-genomic signaling via modulation of intracellular signaling cascades. mSRs are another means besides classical nuclear steroid hormone receptors (SHRs) for steroids to mediate their biological effects. SHRs can produce slow genomic responses or rapid, non-genomic responses in the case of mSRs.
Membrane glucocorticoid receptors (mGRs) are a group of receptors which bind and are activated by glucocorticoids such as cortisol and corticosterone, as well as certain exogenous glucocorticoids such as dexamethasone. Unlike the classical nuclear glucocorticoid receptor (GR), which mediates its effects via genomic mechanisms, mGRs are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades. The identities of the mGRs have yet to be fully elucidated, but are thought to include membrane-associated classical GRs as well as yet-to-be-characterized G protein-coupled receptors (GPCRs). Rapid effects of dexamethasone were found not be reversed by the GR antagonist mifepristone, indicating additional receptors besides just the classical GR.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.
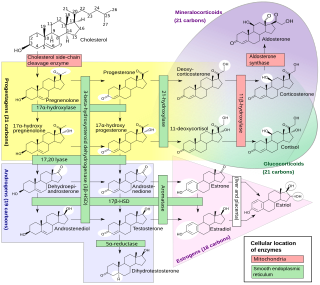
Steroidogenic enzymes are enzymes that are involved in steroidogenesis and steroid biosynthesis. They are responsible for the biosynthesis of the steroid hormones, including sex steroids and corticosteroids, as well as neurosteroids, from cholesterol. Steroidogenic enzymes are most highly expressed in classical steroidogenic tissues, such as the testis, ovary, and adrenal cortex, but are also present in other tissues in the body.
References
- 1 2 3 4 5 Dooley R, Harvey BJ, Thomas W (2012). "Non-genomic actions of aldosterone: from receptors and signals to membrane targets". Mol. Cell. Endocrinol. 350 (2): 223–34. doi:10.1016/j.mce.2011.07.019. PMID 21801805. S2CID 24630510.
- 1 2 3 Groeneweg FL, Karst H, de Kloet ER, Joëls M (2012). "Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling". Mol. Cell. Endocrinol. 350 (2): 299–309. doi:10.1016/j.mce.2011.06.020. PMID 21736918. S2CID 23048944.
- 1 2 de Kloet ER, Karst H, Joëls M (2008). "Corticosteroid hormones in the central stress response: quick-and-slow". Front Neuroendocrinol. 29 (2): 268–72. doi:10.1016/j.yfrne.2007.10.002. PMID 18067954. S2CID 15094025.
- 1 2 Joëls M, Karst H, DeRijk R, de Kloet ER (2008). "The coming out of the brain mineralocorticoid receptor". Trends Neurosci. 31 (1): 1–7. doi:10.1016/j.tins.2007.10.005. PMID 18063498. S2CID 5762688.
- ↑ Funder JW (2005). "The nongenomic actions of aldosterone". Endocr. Rev. 26 (3): 313–21. doi: 10.1210/er.2005-0004 . PMID 15814845.
- 1 2 Mihailidou AS, Funder JW (2005). "Nongenomic effects of mineralocorticoid receptor activation in the cardiovascular system". Steroids. 70 (5–7): 347–51. doi:10.1016/j.steroids.2005.02.004. PMID 15862816. S2CID 43782472.
- 1 2 3 Wendler A, Albrecht C, Wehling M (2012). "Nongenomic actions of aldosterone and progesterone revisited". Steroids. 77 (10): 1002–6. doi:10.1016/j.steroids.2011.12.023. PMID 22285849. S2CID 28968323.
- ↑ Sarabdjitsingh RA, Joëls M, de Kloet ER (2012). "Glucocorticoid pulsatility and rapid corticosteroid actions in the central stress response". Physiol. Behav. 106 (1): 73–80. doi:10.1016/j.physbeh.2011.09.017. PMID 21971364. S2CID 32448734.
- ↑ Groeneweg FL, Karst H, de Kloet ER, Joëls M (2011). "Rapid non-genomic effects of corticosteroids and their role in the central stress response". J. Endocrinol. 209 (2): 153–67. doi: 10.1530/JOE-10-0472 . PMID 21357682.
- ↑ Lösel RM, Wehling M (2008). "Classic versus non-classic receptors for nongenomic mineralocorticoid responses: emerging evidence". Front Neuroendocrinol. 29 (2): 258–67. doi:10.1016/j.yfrne.2007.09.002. PMID 17976711. S2CID 19205927.
- ↑ Thomas W, Harvey BJ (2011). "Mechanisms underlying rapid aldosterone effects in the kidney". Annu. Rev. Physiol. 73: 335–57. doi:10.1146/annurev-physiol-012110-142222. PMID 20809792.
- 1 2 Harvey BJ, Alzamora R, Stubbs AK, Irnaten M, McEneaney V, Thomas W (2008). "Rapid responses to aldosterone in the kidney and colon". J. Steroid Biochem. Mol. Biol. 108 (3–5): 310–7. doi:10.1016/j.jsbmb.2007.09.005. PMID 17951051. S2CID 30197782.
- ↑ Vinson GP, Coghlan JP (2010). "Expanding view of aldosterone action, with an emphasis on rapid action". Clin. Exp. Pharmacol. Physiol. 37 (4): 410–6. doi:10.1111/j.1440-1681.2010.05352.x. PMID 20409082. S2CID 46512893.
- ↑ Meinel S, Gekle M, Grossmann C (2014). "Mineralocorticoid receptor signaling: crosstalk with membrane receptors and other modulators". Steroids. 91: 3–10. doi:10.1016/j.steroids.2014.05.017. PMID 24928729. S2CID 35896263.