
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

Ghrelin is a hormone produced by enteroendocrine cells of the gastrointestinal tract, especially the stomach, and is often called a "hunger hormone" because it increases the drive to eat. Blood levels of ghrelin are highest before meals when hungry, returning to lower levels after mealtimes. Ghrelin may help prepare for food intake by increasing gastric motility and stimulating the secretion of gastric acid.

Motilin is a 22-amino acid polypeptide hormone in the motilin family that, in humans, is encoded by the MLN gene.

Agouti-related protein (AgRP), also called agouti-related peptide, is a neuropeptide produced in the brain by the AgRP/NPY neuron. It is synthesized in neuropeptide Y (NPY)-containing cell bodies located in the ventromedial part of the arcuate nucleus in the hypothalamus. AgRP is co-expressed with NPY and acts to increase appetite and decrease metabolism and energy expenditure. It is one of the most potent and long-lasting of appetite stimulators. In humans, the agouti-related peptide is encoded by the AGRP gene.
Growth hormone–releasing hormone (GHRH), also known as somatocrinin or by several other names in its endogenous forms and as somatorelin (INN) in its pharmaceutical form, is a releasing hormone of growth hormone (GH). It is a 44-amino acid peptide hormone produced in the arcuate nucleus of the hypothalamus.

The follicle-stimulating hormone receptor or FSH receptor (FSHR) is a transmembrane receptor that interacts with the follicle-stimulating hormone (FSH) and represents a G protein-coupled receptor (GPCR). Its activation is necessary for the hormonal functioning of FSH. FSHRs are found in the ovary, testis, and uterus.

The luteinizing hormone/choriogonadotropin receptor (LHCGR), also lutropin/choriogonadotropin receptor (LCGR) or luteinizing hormone receptor (LHR) is a transmembrane receptor found predominantly in the ovary and testis, but also many extragonadal organs such as the uterus and breasts. The receptor interacts with both luteinizing hormone (LH) and chorionic gonadotropins and represents a G protein-coupled receptor (GPCR). Its activation is necessary for the hormonal functioning during reproduction.
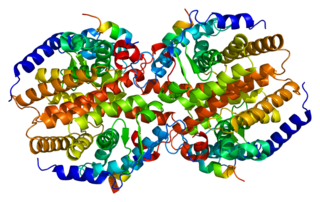
The constitutive androstane receptor (CAR) also known as nuclear receptor subfamily 1, group I, member 3 is a protein that in humans is encoded by the NR1I3 gene. CAR is a member of the nuclear receptor superfamily and along with pregnane X receptor (PXR) functions as a sensor of endobiotic and xenobiotic substances. In response, expression of proteins responsible for the metabolism and excretion of these substances is upregulated. Hence, CAR and PXR play a major role in the detoxification of foreign substances such as drugs.

The glucagon-like peptide-1 receptor (GLP1R) is a receptor protein found on beta cells of the pancreas and on neurons of the brain. It is involved in the control of blood sugar level by enhancing insulin secretion. In humans it is synthesised by the gene GLP1R, which is present on chromosome 6. It is a member of the glucagon receptor family of G protein-coupled receptors. GLP1R is composed of two domains, one extracellular (ECD) that binds the C-terminal helix of GLP-1, and one transmembrane (TMD) domain that binds the N-terminal region of GLP-1. In the TMD domain there is a fulcrum of polar residues that regulates the biased signaling of the receptor while the transmembrane helical boundaries and extracellular surface are a trigger for biased agonism.

Growth hormone-releasing peptide 6 (GHRP-6), also known as growth hormone-releasing hexapeptide, is one of several synthetic met-enkephalin analogues that include unnatural D-amino acids, were developed for their growth hormone-releasing activity and are called growth hormone secretagogues. They lack opioid activity but are potent stimulators of growth hormone (GH) release. These secretagogues are distinct from growth hormone releasing hormone (GHRH) in that they share no sequence relation and derive their function through activation of a completely different receptor. This receptor was originally called the growth hormone secretagogue receptor (GHSR), but due to subsequent discoveries, the hormone ghrelin is now considered the receptor's natural endogenous ligand, and it has been renamed as the ghrelin receptor. Therefore, these GHSR agonists act as synthetic ghrelin mimetics.

The Gs alpha subunit is a subunit of the heterotrimeric G protein Gs that stimulates the cAMP-dependent pathway by activating adenylyl cyclase. Gsα is a GTPase that functions as a cellular signaling protein. Gsα is the founding member of one of the four families of heterotrimeric G proteins, defined by the alpha subunits they contain: the Gαs family, Gαi/Gαo family, Gαq family, and Gα12/Gα13 family. The Gs-family has only two members: the other member is Golf, named for its predominant expression in the olfactory system. In humans, Gsα is encoded by the GNAS complex locus, while Golfα is encoded by the GNAL gene.

G protein-coupled receptor 119 also known as GPR119 is a G protein-coupled receptor that in humans is encoded by the GPR119 gene.

G-protein coupled receptor 3 is a protein that in humans is encoded by the GPR3 gene. The protein encoded by this gene is a member of the G protein-coupled receptor family of transmembrane receptors and is involved in signal transduction.

Pancreatic polypeptide receptor 1, also known as Neuropeptide Y receptor type 4, is a protein that in humans is encoded by the PPYR1 gene.

Ibutamoren (INN) is a potent, long-acting, orally-active, selective, and non-peptide agonist of the ghrelin receptor and a growth hormone secretagogue, mimicking the growth hormone (GH)-stimulating action of the endogenous hormone ghrelin. It has been shown to increase the secretion of several hormones including GH and insulin-like growth factor 1 (IGF-1) and produces sustained increases in the plasma levels of these hormones without affecting cortisol levels.
Cyril Y. Bowers, M.D., emeritus professor of medicine at Tulane University School of Medicine, attended medical school at the University of Oregon and did an internship at the University of Washington. He then studied biochemistry at Cornell University and attended the postgraduate school of medicine at the University of Pennsylvania. From 1961-2004 he was the director of the Section of Endocrinology & Metabolism in the department of medicine at Tulane University School of Medicine. Bowers has served on the editorial board of several endocrine journals, was a member of the National Institute of Diabetes and Digestive and Kidney Diseases Study Section for eight years and has written over 400 articles in peer-reviewed journals, including chapters in books and over 200 abstracts.
Growth hormone secretagogues or GH secretagogues (GHSs) are a class of drugs which act as secretagogues of growth hormone (GH). They include agonists of the ghrelin/growth hormone secretagogue receptor (GHSR), such as ghrelin (lenomorelin), pralmorelin (GHRP-2), GHRP-6, examorelin (hexarelin), ipamorelin, and ibutamoren (MK-677), and agonists of the growth hormone-releasing hormone receptor (GHRHR), such as growth hormone-releasing hormone, CJC-1295, sermorelin, and tesamorelin.

Pralmorelin (INN), also known as pralmorelin hydrochloride (JAN) and pralmorelin dihydrochloride (USAN), as well as, notably, growth hormone-releasing peptide 2 (GHRP-2), is a growth hormone secretagogue (GHS) used as a diagnostic agent that is marketed by Kaken Pharmaceutical in Japan in a single-dose formulation for the assessment of growth hormone deficiency (GHD).

Ipamorelin (INN) (developmental code name NNC 26-0161) is a peptide selective agonist of the ghrelin/growth hormone secretagogue receptor (GHS) and a growth hormone secretagogue. It is a pentapeptide with the amino acid sequence Aib-His-D-2-Nal-D-Phe-Lys-NH2 that was derived from GHRP-1.

Examorelin (INN) (developmental code names EP-23905, MF-6003), also known as hexarelin, is a potent, synthetic, peptidic, orally-active, centrally-penetrant, and highly selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR) and a growth hormone secretagogue which was developed by Mediolanum Farmaceutici. It is a hexapeptide with the amino acid sequence His-D-2-methyl-Trp-Ala-Trp-D-Phe-Lys-NH2 which was derived from GHRP-6. These GH-releasing peptides have no sequence similarity to ghrelin, but mimic ghrelin by acting as agonists at the ghrelin receptor.





















