Hyperthyroidism is the condition that occurs due to excessive production of thyroid hormones by the thyroid gland. Thyrotoxicosis is the condition that occurs due to excessive thyroid hormone of any cause and therefore includes hyperthyroidism. It is noted that thyrotoxicosis is related to hyper-kinetic movement disorders including chorea and myoclonus. Some, however, use the terms interchangeably. Signs and symptoms vary between people and may include irritability, muscle weakness, sleeping problems, a fast heartbeat, heat intolerance, diarrhea, enlargement of the thyroid, hand tremor, and weight loss. Symptoms are typically less severe in the elderly and during pregnancy. An uncommon complication is thyroid storm in which an event such as an infection results in worsening symptoms such as confusion and a high temperature and often results in death. The opposite is hypothyroidism, when the thyroid gland does not make enough thyroid hormone.

Graves' disease, also known as toxic diffuse goiter, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyroid. Signs and symptoms of hyperthyroidism may include irritability, muscle weakness, sleeping problems, a fast heartbeat, poor tolerance of heat, diarrhea and unintentional weight loss. Other symptoms may include thickening of the skin on the shins, known as pretibial myxedema, and eye bulging, a condition caused by Graves' ophthalmopathy. About 25 to 30% of people with the condition develop eye problems.

Thyrotropin-releasing hormone (TRH) is a hypophysiotropic hormone produced by neurons in the hypothalamus that stimulates the release of thyroid-stimulating hormone (TSH) and prolactin from the anterior pituitary.
Thyroid-stimulating hormone (also known as thyrotropin, thyrotropic hormone, or abbreviated TSH) is a pituitary hormone that stimulates the thyroid gland to produce thyroxine (T4), and then triiodothyronine (T3) which stimulates the metabolism of almost every tissue in the body. It is a glycoprotein hormone produced by thyrotrope cells in the anterior pituitary gland, which regulates the endocrine function of the thyroid.

Thyroid disease is a medical condition that affects the function of the thyroid gland. The thyroid gland is located at the front of the neck and produces thyroid hormones that travel through the blood to help regulate many other organs, meaning that it is an endocrine organ. These hormones normally act in the body to regulate energy use, infant development, and childhood development.

Toxic multinodular goiter (TMNG), also known as multinodular toxic goiter (MNTG), is an active multinodular goiter associated with hyperthyroidism.
Thyroid function tests (TFTs) is a collective term for blood tests used to check the function of the thyroid. TFTs may be requested if a patient is thought to suffer from hyperthyroidism or hypothyroidism, or to monitor the effectiveness of either thyroid-suppression or hormone replacement therapy. It is also requested routinely in conditions linked to thyroid disease, such as atrial fibrillation and anxiety disorder.

The follicle-stimulating hormone receptor or FSH receptor (FSHR) is a transmembrane receptor that interacts with the follicle-stimulating hormone (FSH) and represents a G protein-coupled receptor (GPCR). Its activation is necessary for the hormonal functioning of FSH. FSHRs are found in the ovary, testis, and uterus.

The luteinizing hormone/choriogonadotropin receptor (LHCGR), also lutropin/choriogonadotropin receptor (LCGR) or luteinizing hormone receptor (LHR) is a transmembrane receptor found predominantly in the ovary and testis, but also many extragonadal organs such as the uterus and breasts. The receptor interacts with both luteinizing hormone (LH) and chorionic gonadotropins and represents a G protein-coupled receptor (GPCR). Its activation is necessary for the hormonal functioning during reproduction.

Thyrotropin-releasing hormone receptor (TRHR) is a G protein-coupled receptor which binds thyrotropin-releasing hormone.

The hypothalamic–pituitary–thyroid axis is part of the neuroendocrine system responsible for the regulation of metabolism and also responds to stress.

The sodium/iodide cotransporter, also known as the sodium/iodide symporter (NIS), is a protein that in humans is encoded by the SLC5A5 gene. It is a transmembrane glycoprotein with a molecular weight of 87 kDa and 13 transmembrane domains, which transports two sodium cations (Na+) for each iodide anion (I−) into the cell. NIS mediated uptake of iodide into follicular cells of the thyroid gland is the first step in the synthesis of thyroid hormone.

The testicular receptor 2 (TR2) also known as NR2C1 is protein that in humans is encoded by the NR2C1 gene. TR2 is a member of the nuclear receptor family of transcription factors.

Thyroid hormone receptor beta (TR-beta) also known as nuclear receptor subfamily 1, group A, member 2 (NR1A2), is a nuclear receptor protein that in humans is encoded by the THRB gene.

Somatostatin receptor type 1 is a protein that in humans is encoded by the SSTR1 gene.
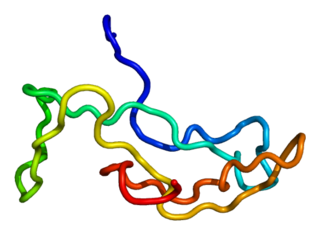
Glycoprotein hormones, alpha polypeptide is a protein that in humans is encoded by the CGA gene.

Immunoglobulin superfamily, member 1 is a plasma membrane glycoprotein encoded by the IGSF1 gene, which maps to the X chromosome in humans and other mammalian species.

Thyroid hormones are any hormones produced and released by the thyroid gland, namely triiodothyronine (T3) and thyroxine (T4). They are tyrosine-based hormones that are primarily responsible for regulation of metabolism. T3 and T4 are partially composed of iodine. A deficiency of iodine leads to decreased production of T3 and T4, enlarges the thyroid tissue and will cause the disease known as simple goitre.

Thyroid stimulating hormone, beta also known as TSHB is a protein which in humans is encoded by the TSHB gene.
Antithyroid autoantibodies (or simply antithyroid antibodies) are autoantibodies targeted against one or more components on the thyroid. The most clinically relevant anti-thyroid autoantibodies are anti-thyroid peroxidase antibodies (anti-TPO antibodies, TPOAb), thyrotropin receptor antibodies (TRAb) and thyroglobulin antibodies (TgAb). TRAb's are subdivided into activating, blocking and neutral antibodies, depending on their effect on the TSH receptor. Anti-sodium/iodide (Anti–Na+/I−) symporter antibodies are a more recent discovery and their clinical relevance is still unknown. Graves' disease and Hashimoto's thyroiditis are commonly associated with the presence of anti-thyroid autoantibodies. Although there is overlap, anti-TPO antibodies are most commonly associated with Hashimoto's thyroiditis and activating TRAb's are most commonly associated with Graves' disease. Thyroid microsomal antibodies were a group of anti-thyroid antibodies; they were renamed after the identification of their target antigen (TPO).