Physiology
Hormone levels
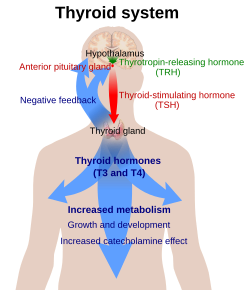
TSH (with a half-life of about an hour) stimulates the thyroid gland to secrete the hormone thyroxine (T4), which has only a slight effect on metabolism. T4 is converted to triiodothyronine (T3), which is the active hormone that stimulates metabolism. About 80% of this conversion is in the liver and other organs, and 20% in the thyroid itself. [1]
TSH is secreted throughout life but particularly reaches high levels during the periods of rapid growth and development, as well as in response to stress.
The hypothalamus, in the base of the brain, produces thyrotropin-releasing hormone (TRH). TRH stimulates the anterior pituitary gland to produce TSH.
Somatostatin is also produced by the hypothalamus, and has an opposite effect on the pituitary production of TSH, decreasing or inhibiting its release.
The concentration of thyroid hormones (T3 and T4) in the blood regulates the pituitary release of TSH; when T3 and T4 concentrations are low, the production of TSH is increased, and, conversely, when T3 and T4 concentrations are high, TSH production is decreased. This is an example of a negative feedback loop. [5] Any inappropriateness of measured values, for instance a low-normal TSH together with a low-normal T4 may signal tertiary (central) disease and a TSH to TRH pathology. Elevated reverse T3 (RT3) together with low-normal TSH and low-normal T3, T4 values, which is regarded as indicative for euthyroid sick syndrome, may also have to be investigated for chronic subacute thyroiditis (SAT) with output of subpotent hormones. Absence of antibodies in patients with diagnoses of an autoimmune thyroid in their past would always be suspicious for development to SAT even in the presence of a normal TSH because there is no known recovery from autoimmunity.
For clinical interpretation of laboratory results it is important to acknowledge that TSH is released in a pulsatile manner [6] [7] [8] resulting in both circadian and ultradian rhythms of its serum concentrations. [9]
Subunits
TSH is a glycoprotein and consists of two subunits, the alpha and the beta subunit.
- The α (alpha) subunit (i.e., chorionic gonadotropin alpha) is nearly identical to that of human chorionic gonadotropin (hCG), luteinizing hormone (LH), and follicle-stimulating hormone (FSH). The α subunit is thought to be the effector region responsible for stimulation of adenylate cyclase (involved the generation of cAMP). [10] The α chain has a 92-amino acid sequence.
- The β (beta) subunit (TSHB) is unique to TSH, and therefore determines its receptor specificity. [11] The β chain has a 118-amino acid sequence.
The TSH receptor
The TSH receptor is found mainly on thyroid follicular cells. [12] Stimulation of the receptor increases T3 and T4 production and secretion. This occurs through stimulation of six steps in thyroid hormone synthesis: (1) Up-regulating the activity of the sodium-iodide symporter (NIS) on the basolateral membrane of thyroid follicular cells, thereby increasing intracellular concentrations of iodine (iodine trapping). (2) Stimulating iodination of thyroglobulin in the follicular lumen, a precursor protein of thyroid hormone. (3) Stimulating the conjugation of iodinated tyrosine residues. This leads to the formation of thyroxine (T4) and triiodothyronine (T3) that remain attached to the thyroglobulin protein. (4) Increased endocytocis of the iodinated thyroglobulin protein across the apical membrane back into the follicular cell. (5) Stimulation of proteolysis of iodinated thyroglobulin to form free thyroxine (T4) and triiodothyronine (T3). (6) Secretion of thyroxine (T4) and triiodothyronine (T3) across the basolateral membrane of follicular cells to enter the circulation. This occurs by an unknown mechanism. [13]
Stimulating antibodies to the TSH receptor mimic TSH and cause Graves' disease. In addition, hCG shows some cross-reactivity to the TSH receptor and therefore can stimulate production of thyroid hormones. In pregnancy, prolonged high concentrations of hCG can produce a transient condition termed gestational hyperthyroidism. [14] This is also the mechanism of trophoblastic tumors increasing the production of thyroid hormones.[ citation needed ]