Related Research Articles

The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the ligand to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.

Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications, are drugs that inhibit or prevent the activity of the immune system.

A monoclonal antibody is an antibody produced from a cell lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
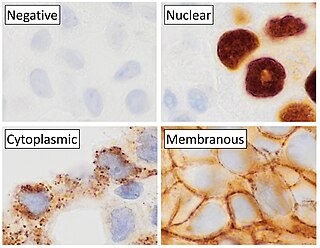
Immunohistochemistry (IHC) is a form of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells and tissue, by exploiting the principle of antibodies binding specifically to antigens in biological tissues. Albert Hewett Coons, Ernest Berliner, Norman Jones and Hugh J Creech was the first to develop immunofluorescence in 1941. This led to the later development of immunohistochemistry.

Hybridoma technology is a method for producing large numbers of identical antibodies. This process starts by injecting a mouse with an antigen that provokes an immune response. A type of white blood cell, the B cell, produces antibodies that bind to the injected antigen. These antibody producing B-cells are then harvested from the mouse and, in turn, fused with immortal myeloma cancer cells, to produce a hybrid cell line called a hybridoma, which has both the antibody-producing ability of the B-cell and the longevity and reproductivity of the myeloma. The hybridomas can be grown in culture, each culture starting with one viable hybridoma cell, producing cultures each of which consists of genetically identical hybridomas which produce one antibody per culture (monoclonal) rather than mixtures of different antibodies (polyclonal). The myeloma cell line that is used in this process is selected for its ability to grow in tissue culture and for an absence of antibody synthesis. In contrast to polyclonal antibodies, which are mixtures of many different antibody molecules, the monoclonal antibodies produced by each hybridoma line are all chemically identical.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology.
Heterophile antibodies are antibodies induced by external antigens.

Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system kills a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.
Theralizumab is an immunomodulatory drug developed by Thomas Hünig of the University of Würzburg. It was withdrawn from development after inducing severe inflammatory reactions as well as chronic organ failure in the first-in-human study by Parexel in London in March 2006. The developing company, TeGenero Immuno Therapeutics, went bankrupt later that year. The commercial rights were then acquired by a Russian startup, TheraMAB. The drug was renamed TAB08. Phase I and II clinical trials have been completed for arthritis and clinical trials have been initiated for cancer.
Humanized antibodies are antibodies from non-human species whose protein sequences have been modified to increase their similarity to antibody variants produced naturally in humans. The process of "humanization" is usually applied to monoclonal antibodies developed for administration to humans. Humanization can be necessary when the process of developing a specific antibody involves generation in a non-human immune system. The protein sequences of antibodies produced in this way are partially distinct from homologous antibodies occurring naturally in humans, and are therefore potentially immunogenic when administered to human patients. The International Nonproprietary Names of humanized antibodies end in -zumab, as in omalizumab.
The nomenclature of monoclonal antibodies is a naming scheme for assigning generic, or nonproprietary, names to monoclonal antibodies. An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus. Monoclonal antibodies are those that were produced in identical cells, often artificially, and so share the same target object. They have a wide range of applications including medical uses.

Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective, to induce a specific cell signal, to cause the immune system to attack specific cells, or to bring a drug to a specific cell type.
Technetium (99mTc) arcitumomab is a drug used for the diagnostic imaging of colorectal cancers, marketed by Immunomedics. It consists of the Fab' fragment of a monoclonal antibody and a radionuclide, technetium-99m.
Minretumomab (CC49) is a mouse monoclonal antibody that was designed for the treatment of cancers that express the TAG-72 antigen. This includes breast, colon, lung, and pancreatic cancers. Apparently, it never got past Phase I clinical trials for this purpose.

Anti-double stranded DNA (Anti-dsDNA) antibodies are a group of anti-nuclear antibodies (ANA) the target antigen of which is double stranded DNA. Blood tests such as enzyme-linked immunosorbent assay (ELISA) and immunofluorescence are routinely performed to detect anti-dsDNA antibodies in diagnostic laboratories. They are highly diagnostic of systemic lupus erythematosus (SLE) and are implicated in the pathogenesis of lupus nephritis.
Siltuximab is a chimeric monoclonal antibody. It binds to interleukin-6. Siltuximab has been investigated for the treatment of neoplastic diseases: metastatic renal cell cancer, prostate cancer, other types of cancer, and for Castleman's disease.

A trifunctional antibody is a monoclonal antibody with binding sites for two different antigens, typically CD3 and a tumor antigen, making it a type of bispecific monoclonal antibody. In addition, its intact Fc-part can bind to an Fc receptor on accessory cells like conventional monospecific antibodies. The net effect is that this type of drug links T cells and monocytes/macrophages, natural killer cells, dendritic cells or other Fc receptor expressing cells to the tumor cells, leading to their destruction.
Short Course Immune Induction Therapy or SCIIT, is a therapeutic strategy employing rapid, specific, short term-modulation of the immune system using a therapeutic agent to induce T-cell non-responsiveness, also known as operational tolerance. As an alternative strategy to immunosuppression and antigen-specific tolerance inducing therapies, the primary goal of SCIIT is to re-establish or induce peripheral immune tolerance in the context of autoimmune disease and transplant rejection through the use of biological agents. In recent years, SCIIT has received increasing attention in clinical and research settings as an alternative to immunosuppressive drugs currently used in the clinic, drugs which put the patients at risk of developing infection, cancer, and cardiovascular disease.
MDX-1097 is a monoclonal antibody therapy that has most recently been assessed in a Phase IIb clinical trial (2023) in conjunction with lenalidomide and dexamethasone as a treatment for multiple myeloma, a type of white blood cell cancer. MDX-1097 was originally developed by scientists at Immune System Therapeutics Ltd. In 2015, Haemalogix Ltd acquired the rights to MDX-1097 and are now taking it through clinical testing.
Passive antibody therapy, also called serum therapy, is a subtype of passive immunotherapy that administers antibodies to target and kill pathogens or cancer cells. It is designed to draw support from foreign antibodies that are donated from a person, extracted from animals, or made in the laboratory to elicit an immune response instead of relying on the innate immune system to fight disease. It has a long history from the 18th century for treating infectious diseases and is now a common cancer treatment. The mechanism of actions include: antagonistic and agonistic reaction, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC).
References
- ↑ "Antibody-3F8". Sloan-Kettering. Archived from the original on 2015-09-18.
- ↑ MedlinePlus Encyclopedia : Cerebral spinal fluid (CSF) collection
- ↑ "HAMA: Human Anti-Mouse Antibodies". Patients Against Lymphoma. February 18, 2013.[ unreliable medical source? ]
- ↑ Azinovic, Ignacio; DeNardo, Gerald L.; Lamborn, Kathleen R.; Mirick, Gary; Goldstein, Desiree; Bradt, Bonnie M.; DeNardo, Sally J. (2006). "Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignancies". Cancer Immunology, Immunotherapy. 55 (12): 1451–8. doi:10.1007/s00262-006-0148-4. PMC 11030743 . PMID 16496145. S2CID 1528225.
- 1 2 Klee, George G. (2000). "Human Anti-Mouse Antibodies". Archives of Pathology & Laboratory Medicine. 124 (6): 921–3. doi:10.5858/2000-124-0921-HAMA. PMID 10835540.
- ↑ Marx, Uwe; Embleton, M. Jim; Fischer, René; Gruber, Franz P.; Hansson, Ulrika; Heuer, Joachim; de Leeuw, Wim A.; Logtenberg, Ton; Merz, Wolfram; Portetelle, Daniel; Romette, John-Louis; Straughan, Donald W. (March 1997). "Monoclonal Antibody Production". ATLA. 25 (2): 121–37. INIST 2645205.
- ↑ Gruber, Franz P.; Hartung, Thomas (2004). "Alternatives to Animal Experimentation in Basic Research" (PDF). ALTEX. 21 (Suppl 1): 3–31. doi:10.14573/altex.2004.suppl.3. PMID 15586255.
- ↑ Darwish, IA (2006). "Immunoassay Methods and their Applications in Pharmaceutical Analysis: Basic Methodology and Recent Advances". Int J Biomed Sci. 2 (3): 217–35. PMC 3614608 . PMID 23674985.
- ↑ Tate, J; Ward, G (2004). "Interferences in immunoassay". Clin Biochem Rev. 25 (2): 105–20. PMC 1904417 . PMID 18458713.