Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.
Rimonabant (also known as SR141716; trade names Acomplia, Zimulti) is an anorectic antiobesity drug approved in Europe in 2006 but was withdrawn worldwide in 2008 due to serious psychiatric side effects; it was never approved in the United States. Rimonabant is an inverse agonist for the cannabinoid receptor CB1 and was first-in-class for clinical development.

Vasopressin V1b receptor (V1BR) also known as vasopressin 3 receptor (VPR3) or antidiuretic hormone receptor 1B is a protein that in humans is encoded by the AVPR1B gene.
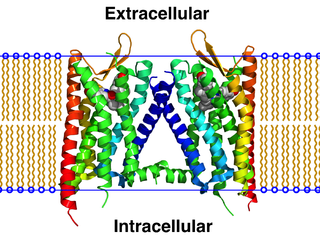
The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the OPRK1 gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction.
The galanin receptor is a G protein-coupled receptor, or metabotropic receptor which binds galanin.

The oxytocin receptor, also known as OXTR, is a protein which functions as receptor for the hormone and neurotransmitter oxytocin. In humans, the oxytocin receptor is encoded by the OXTR gene which has been localized to human chromosome 3p25.
The orexin receptor (also referred to as the hypocretin receptor) is a G-protein-coupled receptor that binds the neuropeptide orexin. There are two variants, OX1 and OX2, each encoded by a different gene (HCRTR1, HCRTR2).

FG-7142 (ZK-31906) is a drug which acts as a partial inverse agonist at the benzodiazepine allosteric site of the GABAA receptor. It has anorectic, anxiogenic and pro-convulsant effects. It also increases release of acetylcholine and noradrenaline, and improves memory retention in animal studies.

Antalarmin (CP-156,181) is a drug that acts as a CRH1 antagonist.

Surinabant (SR147778) is a cannabinoid receptor type 1 antagonist developed by Sanofi-Aventis. It is being investigated as a potential treatment for nicotine addiction, to assist smoking cessation. It may also be developed as an anorectic drug to assist with weight loss, however there are already several CB1 antagonists or inverse agonists on the market or under development for this application, so surinabant is at present mainly being developed as an anti-smoking drug, with possible application in the treatment of other addictive disorders such as alcoholism. Other potential applications such as treatment of ADHD have also been proposed.
Amibegron (SR-58,611A) was a drug developed by Sanofi-Aventis which acts as a selective agonist for the β3 adrenergic receptor. It is the first orally active β3 agonist developed that is capable of entering the central nervous system, and has antidepressant and anxiolytic effects.

Satavaptan is a vasopressin-2 receptor antagonist which was investigation by Sanofi-Aventis and was under development for the treatment of hyponatremia. It was also being studied for the treatment of ascites. Development was discontinued in 2009.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.
A vasopressin receptor antagonist (VRA) is an agent that interferes with action at the vasopressin receptors. Most commonly VRAs are used in the treatment of hyponatremia, especially in patients with congestive heart failure, liver cirrhosis or SIADH.

WAY-267464 is a potent, selective, non-peptide agonist for the oxytocin receptor, with negligible affinity for the vasopressin receptors. Contradictorily however, though originally described as selective for the oxytocin receptor and lacking affinity for the vasopressin receptors, it has since been reported to also act as a potent vasopressin V1A receptor antagonist. WAY-267464 has been shown to cross the blood–brain barrier to a significantly greater extent than exogenously applied oxytocin, and in animal tests produces centrally-mediated oxytocinergic actions such as anxiolytic effects, but with no antidepressant effect evident. It was developed by a team at Ferring Pharmaceuticals. WAY-267464 was under investigation for the potential clinical treatment of anxiety disorders by Wyeth, and reached the preclinical stage of development, but no development has been reported as of 2011.

Epelsiban is an orally bioavailable drug which acts as a selective and potent oxytocin receptor antagonist. It was initially developed by GlaxoSmithKline (GSK) for the treatment of premature ejaculation in men and then as an agent to enhance embryo or blastocyst implantation in women undergoing embryo or blastocyst transfer associated with in vitro fertilization (IVF)., and was also investigated for use in the treatment of adenomyosis.
Drinabant (INN; AVE-1625) is a drug that acts as a selective CB1 receptor antagonist, which was under investigation varyingly by Sanofi-Aventis as a treatment for obesity, schizophrenia, Alzheimer's disease, Parkinson's disease, and nicotine dependence. Though initially studied as a potential treatment for a variety of different medical conditions, Sanofi-Aventis eventually narrowed down the therapeutic indications of the compound to just appetite suppression. Drinabant reached phase IIb clinical trials for this purpose in the treatment of obesity but was shortly thereafter discontinued, likely due to the observation of severe psychiatric side effects including anxiety, depression, and thoughts of suicide in patients treated with the now-withdrawn rimonabant, another CB1 antagonist that was also under development by Sanofi-Aventis.

SRX246, also known as API-246, is a small-molecule, centrally-active, highly-selective vasopressin V1A receptor antagonist which is under investigation by Azevan Pharmaceuticals for the treatment of affective and anger disorders. It is an azetidinone derivative, and was developed from LY-307174 as a lead compound. A phase II activity trial of the drug in the treatment of adults with intermittent explosive disorder is ongoing. It is also being studied for the treatment of post-traumatic stress disorder.
TS-121 (THY1773) is an orally active, selective vasopressin V1B receptor antagonist which is under development by Taisho Pharmaceutical for the adjunctive treatment of major depressive disorder. As of May 2017, it is in phase II clinical trials for this indication.
ABT-436 is an orally active, highly selective vasopressin V1B receptor antagonist which was under development by Abbott Laboratories and AbbVie for the treatment of major depressive disorder, anxiety disorders, and alcoholism but was discontinued. It reached phase II clinical trials prior to the discontinuation of its development.