
A selective progesterone receptor modulator (SPRM) is an agent that acts on the progesterone receptor (PR), the biological target of progestogens like progesterone. A characteristic that distinguishes such substances from full receptor agonists and full antagonists is that their action differs in different tissues, i.e. agonist in some tissues while antagonist in others. This mixed profile of action leads to stimulation or inhibition in tissue-specific manner, which further raises the possibility of dissociating undesirable adverse effects from the development of synthetic PR-modulator drug candidates.

Ritanserin, also known by its developmental code name R-55667, is a serotonin antagonist medication described as an anxiolytic, antidepressant, antiparkinsonian agent, and antihypertensive agent. It was chiefly investigated as a drug to treat insomnia, especially to enhance sleep quality by significantly increasing slow wave sleep by virtue of potent and concomitant 5-HT2A and 5-HT2C receptor antagonism.
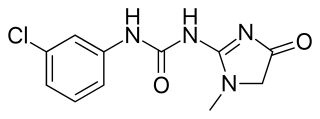
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5, and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.

The 5-HT3 antagonists, informally known as "setrons", are a class of drugs that act as receptor antagonists at the 5-HT3 receptor, a subtype of serotonin receptor found in terminals of the vagus nerve and in certain areas of the brain. With the notable exceptions of alosetron and cilansetron, which are used in the treatment of irritable bowel syndrome, all 5-HT3 antagonists are antiemetics, used in the prevention and treatment of nausea and vomiting. They are particularly effective in controlling the nausea and vomiting produced by cancer chemotherapy and are considered the gold standard for this purpose.

SB-277,011A is a drug which acts as a potent and selective dopamine D3 receptor antagonist, which is around 80–100 times selective for D3 over D2, and lacks any partial agonist activity.

The adenosine A2B receptor, also known as ADORA2B, is a G-protein coupled adenosine receptor, and also denotes the human adenosine A2b receptor gene which encodes it.

Orexin receptor type 2 (Ox2R or OX2), also known as hypocretin receptor type 2 (HcrtR2), is a protein that in humans is encoded by the HCRTR2 gene. It should not be confused for the protein CD200R1 which shares the alias OX2R but is a distinct, unrelated gene located on the human chromosome 3.

Dopamine receptor D3 is a protein that in humans is encoded by the DRD3 gene.

SCH-58261 is a drug which acts as a potent and selective antagonist for the adenosine receptor A2A, with more than 50x selectivity for A2A over other adenosine receptors. It has been used to investigate the mechanism of action of caffeine, which is a mixed A1 / A2A antagonist, and has shown that the A2A receptor is primarily responsible for the stimulant and ergogenic effects of caffeine, but blockade of both A1 and A2A receptors is required to accurately replicate caffeine's effects in animals. SCH-58261 has also shown antidepressant, nootropic and neuroprotective effects in a variety of animal models, and has been investigated as a possible treatment for Parkinson's disease.

J-113,397 is an opioid drug which was the first compound found to be a highly selective antagonist for the nociceptin receptor, also known as the ORL-1 receptor. It is several hundred times selective for the ORL-1 receptor over other opioid receptors, and its effects in animals include preventing the development of tolerance to morphine, the prevention of hyperalgesia induced by intracerebroventricular administration of nociceptin, as well as the stimulation of dopamine release in the striatum, which increases the rewarding effects of cocaine, but may have clinical application in the treatment of Parkinson's disease.

Selective glucocorticoid receptor modulators (SEGRMs) and selective glucocorticoid receptor agonists (SEGRAs) formerly known as dissociated glucocorticoid receptor agonists (DIGRAs) are a class of experimental drugs designed to share many of the desirable anti-inflammatory, immunosuppressive, or anticancer properties of classical glucocorticoid drugs but with fewer side effects such as skin atrophy. Although preclinical evidence on SEGRAMs’ anti-inflammatory effects are culminating, currently, the efficacy of these SEGRAMs on cancer are largely unknown.
A vasopressin receptor antagonist (VRA) is an agent that interferes with action at the vasopressin receptors. Most commonly VRAs are used in the treatment of hyponatremia, especially in patients with congestive heart failure, liver cirrhosis or SIADH.
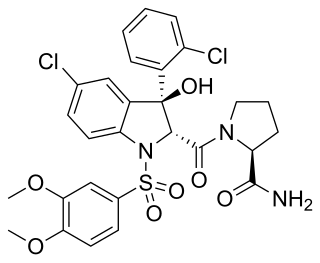
Relcovaptan (SR-49059) is a non-peptide vasopressin receptor antagonist, selective for the V1a subtype. It has shown positive initial results for the treatment of Raynaud's disease and dysmenorrhoea, and as a tocolytic, although it is not yet approved for clinical use.

WAY-267464 is a potent, selective, non-peptide agonist for the oxytocin receptor, with negligible affinity for the vasopressin receptors. Contradictorily however, though originally described as selective for the oxytocin receptor and lacking affinity for the vasopressin receptors, it has since been reported to also act as a potent vasopressin V1A receptor antagonist. WAY-267464 has been shown to cross the blood–brain barrier to a significantly greater extent than exogenously applied oxytocin, and in animal tests produces centrally-mediated oxytocinergic actions such as anxiolytic effects, but with no antidepressant effect evident. It was developed by a team at Ferring Pharmaceuticals. WAY-267464 was under investigation for the potential clinical treatment of anxiety disorders by Wyeth, and reached the preclinical stage of development, but no development has been reported as of 2011.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.
CCR5 receptor antagonists are a class of small molecules that antagonize the CCR5 receptor. The C-C motif chemokine receptor CCR5 is involved in the process by which HIV, the virus that causes AIDS, enters cells. Hence antagonists of this receptor are entry inhibitors and have potential therapeutic applications in the treatment of HIV infections.

Epelsiban is an orally bioavailable drug which acts as a selective and potent oxytocin receptor antagonist. It was initially developed by GlaxoSmithKline (GSK) for the treatment of premature ejaculation in men and then as an agent to enhance embryo or blastocyst implantation in women undergoing embryo or blastocyst transfer associated with in vitro fertilization (IVF)., and was also investigated for use in the treatment of adenomyosis.
5-HT2C receptor agonists are a class of drugs that activate 5-HT2C receptors. They have been investigated for the treatment of a number of conditions including obesity, psychiatric disorders, sexual dysfunction and urinary incontinence.

WB-4101 is a compound which acts as an antagonist at the α1B-adrenergic receptor. It was one of the first selective antagonists developed for this receptor and was invented in 1969, but is still commonly used in research into adrenergic receptors, especially as a lead compound from which to develop more selective drugs.

SRX246, also known as API-246, is a small-molecule, centrally-active, highly-selective vasopressin V1A receptor antagonist which is under investigation by Azevan Pharmaceuticals for the treatment of affective and anger disorders. It is an azetidinone derivative, and was developed from LY-307174 as a lead compound. A phase II activity trial of the drug in the treatment of adults with intermittent explosive disorder is ongoing. It is also being studied for the treatment of post-traumatic stress disorder.

















