
Risperidone, sold under the brand name Risperdal among others, is an atypical antipsychotic used to treat schizophrenia and bipolar disorder, as well as irritability associated with autism. It is taken either by mouth or by injection. The injectable versions are long-acting and last for 2–4 weeks.
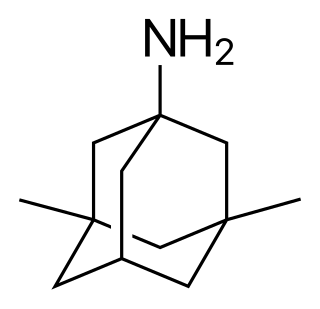
Memantine, sold under the brand name Namenda among others, is a medication used to slow the progression of moderate-to-severe Alzheimer's disease. It is taken by mouth.

Conivaptan, sold under the brand name Vaprisol, is a non-peptide inhibitor of the receptor for anti-diuretic hormone, also called vasopressin. It was approved in 2004 for hyponatremia. The compound was discovered by Astellas and marked in 2006. The drug is now marketed by Cumberland Pharmaceuticals, Inc.

Autism therapies include a wide variety of therapies that help people with autism, or their families. Such methods of therapy seek to aid autistic people in dealing with difficulties and increase their functional independence.

Nelivaptan (INN) is a selective, orally active, non-peptide vasopressin receptor antagonist selective for the V1B subtype. The drug had entered clinical trials for treatment of anxiety and depression. In July 2008, Sanofi-Aventis announced that further development of this drug had been halted.
A vasopressin receptor antagonist (VRA) is an agent that interferes with action at the vasopressin receptors. Most commonly VRAs are used in the treatment of hyponatremia, especially in patients with congestive heart failure, liver cirrhosis or SIADH.

Lixivaptan (VPA-985) is an orally-active, non-peptide, selective vasopressin 2 receptor antagonist being developed as an investigational drug by Palladio Biosciences, Inc. (Palladio), a subsidiary of Centessa Pharmaceuticals plc. As of December 2021, lixivaptan is in Phase III clinical development for the treatment of Autosomal dominant polycystic kidney disease (ADPKD), the most common form of polycystic kidney disease. The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to lixivaptan for the treatment of ADPKD.
An orexin receptor antagonist, or orexin antagonist, is a drug that inhibits the effect of orexin by acting as a receptor antagonist of one (selective orexin receptor antagonist or SORA) or both (dual orexin receptor antagonis or DORA) of the orexin receptors, OX1 and OX2. Medical applications include treatment of sleep disorders such as insomnia.

Aticaprant, also known by its developmental codes JNJ-67953964, CERC-501, and LY-2456302, is a κ-opioid receptor (KOR) antagonist which is under development for the treatment of major depressive disorder. A regulatory application for approval of the medication is expected to be submitted by 2025. Aticaprant is taken by mouth.
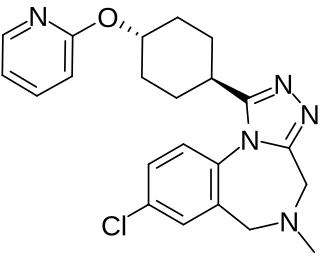
Balovaptan, is a selective small molecule antagonist of the vasopressin V1A receptor which is under development by Roche for the treatment of post-traumatic stress disorder.

Seltorexant, also known by its developmental code names MIN-202 and JNJ-42847922, is an orexin antagonist medication which is under development for the treatment of depression and insomnia. It is a selective antagonist of the orexin OX2 receptor (2-SORA). The medication is taken by mouth.

SRX246, also known as API-246, is a small-molecule, centrally-active, highly-selective vasopressin V1A receptor antagonist which is under investigation by Azevan Pharmaceuticals for the treatment of affective and anger disorders. It is an azetidinone derivative, and was developed from LY-307174 as a lead compound. A phase II activity trial of the drug in the treatment of adults with intermittent explosive disorder is ongoing. It is also being studied for the treatment of post-traumatic stress disorder.

Dextromethorphan/bupropion (DXM/BUP), sold under the brand name Auvelity, is a combination medication for the treatment of major depressive disorder (MDD). Its active components are dextromethorphan (DXM) and bupropion. Patients who stayed on the medication had an average of 11% greater reduction in depressive symptoms than placebo in an FDA approval trial. It is taken as a tablet by mouth.

Brezivaptan is an orally active, selective vasopressin V1B receptor antagonist which is under development by Taisho Pharmaceutical for the adjunctive treatment of major depressive disorder. As of November 2022, it is in phase II clinical trials for this indication.

Ralmitaront is an investigational antipsychotic drug which is undergoing a clinical trial for the treatment of negative symptoms in schizophrenia and schizoaffective disorder. Another clinical trial targeting acute psychotic symptoms of schizophrenia has been terminated due to lack of efficacy. It is a partial agonist of the TAAR1. The medication is being developed by the pharmaceutical company Hoffmann-La Roche. Ralmitaront had completed phase 1 clinical trials.

Vornorexant, also known by its developmental code names ORN-0829 and TS-142, is an orexin antagonist medication which is under development for the treatment of insomnia and sleep apnea. It is a dual orexin OX1 and OX2 receptor antagonist (DORA). The medication is taken by mouth. As of June 2021, vornorexant is in phase 2 clinical trials for insomnia and phase 1 trials for sleep apnea. It is under development by Taisho Pharmaceutical.
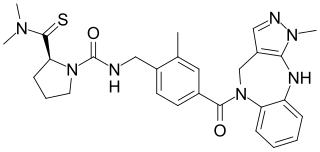
LIT-001 is a small-molecule oxytocin receptor agonist and vasopressin receptor mixed agonist and antagonist that was first described in the literature in 2018. Along with TC OT 39 and WAY-267464, it is one of the first small-molecule oxytocin receptor agonists to have been developed. LIT-001 has greatly improved pharmacokinetic properties relative to oxytocin, reduces social deficits in animal models, and may have potential as a therapeutic agent in the treatment of social disorders like autism in humans.















