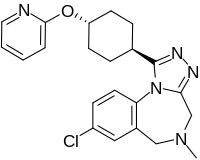
Conivaptan, sold under the brand name Vaprisol, is a non-peptide inhibitor of the receptor for anti-diuretic hormone, also called vasopressin. It was approved in 2004 for hyponatremia. The compound was discovered by Astellas and marked in 2006. The drug is now marketed by Cumberland Pharmaceuticals, Inc.

Autism therapies include a wide variety of therapies that help people with autism, or their families. Such methods of therapy seek to aid autistic people in dealing with difficulties and increase their functional independence.
Ocrelizumab, sold under the brand name Ocrevus, is a medication used for the treatment of multiple sclerosis. It is a humanized anti-CD20 monoclonal antibody. It targets CD20 marker on B lymphocytes and is an immunosuppressive drug. Ocrelizumab binds to an epitope that overlaps with the epitope to which rituximab binds. It is administered by intravenous infusion. The fixed-dose combination ocrelizumab/hyaluronidase is administered by subcutaneous injection.
A vasopressin receptor antagonist (VRA) is an agent that interferes with action at the vasopressin receptors. Most commonly VRAs are used in the treatment of hyponatremia, especially in patients with congestive heart failure, liver cirrhosis or SIADH.

Lixivaptan (VPA-985) is an orally-active, non-peptide, selective vasopressin 2 receptor antagonist being developed as an investigational drug by Palladio Biosciences, Inc. (Palladio), a subsidiary of Centessa Pharmaceuticals plc. As of December 2021, lixivaptan is in Phase III clinical development for the treatment of Autosomal dominant polycystic kidney disease (ADPKD), the most common form of polycystic kidney disease. The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to lixivaptan for the treatment of ADPKD.

Pimavanserin, sold under the brand name Nuplazid, is an atypical antipsychotic which is approved for the treatment of Parkinson's disease psychosis. Unlike other antipsychotics, pimavanserin is not a dopamine receptor antagonist, but rather is a selective inverse agonist of the serotonin 5-HT2A receptor.
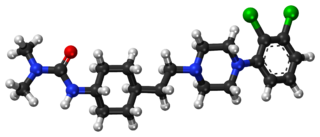
Cariprazine, sold under the brand name Vraylar among others, is an atypical antipsychotic developed by Gedeon Richter, which is used in the treatment of schizophrenia, bipolar mania, bipolar depression, and major depressive disorder. It acts primarily as a D3 and D2 receptor partial agonist, with a preference for the D3 receptor. Cariprazine is also a partial agonist at the serotonin 5-HT1A receptor and acts as an antagonist at 5-HT2B and 5-HT2A receptors, with high selectivity for the D3 receptor. It is taken by mouth. The most prevalent side effects include nausea, mild sedation, fatigue, and dizziness. At higher dosages, there is an increased risk for restlessness, insomnia, and tremors.

Brexpiprazole, sold under the brand name Rexulti among others, is an atypical antipsychotic medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer's disease.

Rapastinel is a novel antidepressant that was under development by Allergan as an adjunctive therapy for the treatment of treatment-resistant depression. It is a centrally active, intravenously administered amidated tetrapeptide that acts as a novel and selective modulator of the NMDA receptor. The drug is a rapid-acting and long-lasting antidepressant as well as robust cognitive enhancer by virtue of its ability to enhance NMDA receptor-mediated signal transduction and synaptic plasticity.

Metadoxine, also known as pyridoxine-pyrrolidone carboxylate, is a drug used to treat chronic and acute alcohol intoxication. Metadoxine accelerates alcohol clearance from the blood.

Basimglurant (INN) is a negative allosteric modulator of the mGlu5 receptor which is under development by Roche and Chugai Pharmaceutical for the treatment of treatment-resistant depression and fragile X syndrome. As of November 2016, it has undergone phase II clinical trials for both of these indications.

SRX246, also known as API-246, is a small-molecule, centrally-active, highly-selective vasopressin V1A receptor antagonist which is under investigation by Azevan Pharmaceuticals for the treatment of affective and anger disorders. It is an azetidinone derivative, and was developed from LY-307174 as a lead compound. A phase II activity trial of the drug in the treatment of adults with intermittent explosive disorder is ongoing. It is also being studied for the treatment of post-traumatic stress disorder.
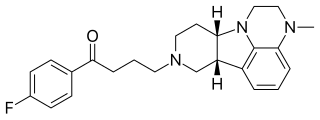
Lumateperone, sold under the brand name Caplyta, is an atypical antipsychotic medication of the butyrophenone class. It is approved for the treatment of schizophrenia as well as bipolar depression, as either monotherapy or adjunctive therapy. It is developed by Intra-Cellular Therapies, licensed from Bristol-Myers Squibb. Lumateperone was approved for medical use in the United States in December 2019 with an initial indication for schizophrenia, and became available in February 2020. It has since demonstrated efficacy in bipolar depression and received FDA approval in December 2021 for depressive episodes associated with both bipolar I and II disorders. Part of the drug shows structural similarity to Pirlindole.

Erdafitinib, sold under the brand name Balversa, is an anti-cancer medication. It is a small molecule inhibitor of fibroblast growth factor receptor (FGFR) used for the treatment of cancer. FGFRs are a subset of tyrosine kinases which are unregulated in some tumors and influence tumor cell differentiation, proliferation, angiogenesis, and cell survival. Astex Pharmaceuticals discovered the drug and licensed it to Janssen Pharmaceuticals for further development.

Brezivaptan is an orally active, selective vasopressin V1B receptor antagonist which is under development by Taisho Pharmaceutical for the adjunctive treatment of major depressive disorder. As of November 2022, it is in phase II clinical trials for this indication.
Satralizumab, sold under the brand name Enspryng, is a humanized monoclonal antibody medication that is used for the treatment of neuromyelitis optica spectrum disorder (NMOSD), a rare autoimmune disease. The drug is being developed by Chugai Pharmaceutical, a subsidiary of Roche.
Psychoplastogens are a group of small molecule drugs that produce rapid and sustained effects on neuronal structure and function, intended to manifest therapeutic benefit after a single administration. Several existing psychoplastogens have been identified and their therapeutic effects demonstrated; several are presently at various stages of development as medications including ketamine, MDMA, scopolamine, and the serotonergic psychedelics, including LSD, psilocin, DMT, and 5-MeO-DMT. Compounds of this sort are being explored as therapeutics for a variety of brain disorders including depression, addiction, and PTSD. The ability to rapidly promote neuronal changes via mechanisms of neuroplasticity was recently discovered as the common therapeutic activity and mechanism of action.
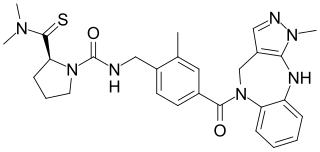
LIT-001 is a small-molecule oxytocin receptor agonist and vasopressin receptor mixed agonist and antagonist that was first described in the literature in 2018. Along with TC OT 39 and WAY-267464, it is one of the first small-molecule oxytocin receptor agonists to have been developed. LIT-001 has greatly improved pharmacokinetic properties relative to oxytocin, reduces social deficits in animal models, and may have potential as a therapeutic agent in the treatment of social disorders like autism in humans.

RG7713, or RG-7713, also known as RO5028442, is a small-molecule vasopression V1A receptor antagonist which is or was under development for the treatment of pervasive developmental disorders or autism. It is administered by intravenous injection.