In finance, a derivative is a contract between a buyer and a seller. The derivative can take various forms, depending on the transaction, but every derivative has the following four elements:
- an item that can or must be bought or sold,
- a future act which must occur,
- a price at which the future transaction must take place, and
- a future date by which the act must take place.

Cefmenoxime is a third-generation cephalosporin antibiotic.

Oxatomide, sold under the brand name Tinset among others, is a antihistamine of the diphenylmethylpiperazine family which is marketed in Europe, Japan, and a number of other countries. It was discovered at Janssen Pharmaceutica in 1975. Oxatomide lacks any anticholinergic effects. In addition to its H1 receptor antagonism, it also possesses antiserotonergic activity similarly to hydroxyzine. Oxatomide was also found to have antiviral activity against Venezuelan equine encephalitis virus (VEEV).

Acetyldihydrocodeine is an opiate derivative discovered in Germany in 1914 and was used as a cough suppressant and analgesic. It is not commonly used, but has activity similar to other opiates. Acetyldihydrocodeine is a very close relative derivative of thebacon, where only the 6-7 bond is unsaturated. Acetyldihydrocodeine can be described as the 6-acetyl derivative of dihydrocodeine and is metabolised in the liver by demethylation and deacetylation to produce dihydromorphine.

Minaprine is a monoamine oxidase inhibitor antidepressant drug that was used in France for the treatment of depression until it was withdrawn from the market in 1996 because it caused convulsions.

Tretoquinol is a beta-adrenergic agonist.
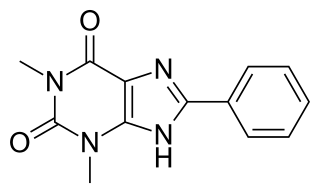
8-Phenyltheophylline (8-phenyl-1,3-dimethylxanthine, 8-PT) is a drug derived from the xanthine family which acts as a potent and selective antagonist for the adenosine receptors A1 and A2A, but unlike other xanthine derivatives has virtually no activity as a phosphodiesterase inhibitor. It has stimulant effects in animals with similar potency to caffeine. Coincidentally 8-phenyltheophylline has also been found to be a potent and selective inhibitor of the liver enzyme CYP1A2 which makes it likely to cause interactions with other drugs which are normally metabolised by CYP1A2.
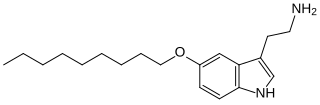
5-(Nonyloxy)tryptamine (5-NOT) is a tryptamine derivative which acts as a selective agonist at the 5-HT1B receptor. Increasing the O-alkoxy chain length in this series gives generally increasing potency and selectivity for 5-HT1B, with highest activity found for the nonyloxy derivative, having a 5-HT1B binding affinity of 1.0 nM, and around 300-fold selectivity over the related 5-HT1A receptor.

WIN 56,098 is a chemical that is considered to be an aminoalkylindole derivative. It is a tricyclic aryl derivative that acts as a competitive antagonist at the CB2 cannabinoid receptor. Its activity at CB1 was significantly less effective. WIN 56,098 failed to antagonize any of the in vivo effects of THC.
BzODZ-EPyr is an indole based synthetic cannabinoid that has been sold as a designer drug in Russia.
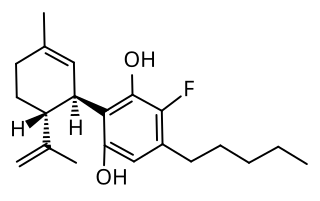
4'-Fluorocannabidiol is a fluorinated cannabidiol derivative that has more potent anxiolytic, antidepressant, antipsychotic and anti-compulsive activity in mice compared to its parent compound. It was first synthesized in 2016, alongside 10-fluorocannabidiol diacetate and 8,9-dihydro-7-fluorocannabidiol, which showed much weaker activity.

17α-Methylprogesterone (17α-MP), or 17α-methylpregn-4-ene-3,20-dione, is a steroidal progestin related to progesterone that was synthesized and characterized in 1949 but was never marketed. Along with ethisterone (1938) and 19-norprogesterone (1951), 17α-MP was one of the earliest derivatives of progesterone to be identified as possessing progestogenic activity. Similarly to progesterone and derivatives like 17α-hydroxyprogesterone and 19-norprogesterone, 17α-MP was found to possess poor oral bioavailability, but showed improved progestogenic activity relative to progesterone when administered via other routes. In addition to its activity as a progestogen, 17α-MP has also been found to possess some antiglucocorticoid activity.
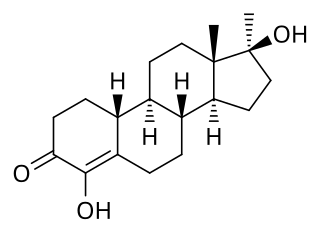
Methylhydroxynandrolone, also known as 4-hydroxy-17α-methyl-19-nortestosterone (HMNT), as well as 4,17β-dihydroxy-17α-methylestr-4-en-3-one, is a synthetic, orally active anabolic–androgenic steroid (AAS) and a 17α-alkylated derivative of nandrolone (19-nortestosterone) which was never marketed. It was first described in 1964 and was studied in the treatment of breast cancer, but was not introduced for clinical use. The drug re-emerged in 2004 when it started being sold on the Internet as a "dietary supplement". MOHN joined other AAS as a controlled substance in the United States on 20 January 2005.
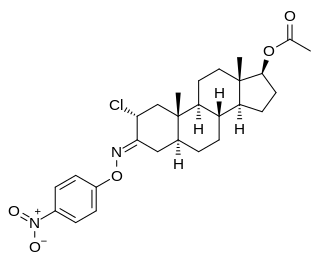
Nisterime acetate (USAN) (developmental code name ORF-9326), also known as 2α-chloro-4,5α-dihydrotestosterone O-(p-nitrophenyl)oxime 17β-acetate or as 2α-chloro-5α-androstan-17β-ol-3-one O-(p-nitrophenyl)oxime 17β-acetate, is a synthetic, orally active anabolic-androgenic steroid (AAS) and a derivative of dihydrotestosterone (DHT) that was developed as a postcoital contraceptive but was never marketed. It is an androgen ester – specifically, the C17α acetate ester of nisterime. Unlike antiprogestogens like mifepristone, nisterime acetate does not prevent implantation and instead induces embryo resorption as well as interrupts the post-implantation stage of pregnancy.

Bromethenmadinone acetate is a progestin medication which was developed in Czechoslovakia and was described in 1970 but was never marketed. Analogues of BMMA include chlormethenmadinone acetate, melengestrol acetate, and methenmadinone acetate.

α-PCyP (α-PyrrolidinoCyclohexanoPhenone) is a stimulant drug of the cathinone class that has been sold online as a designer drug. In a series of alpha-substituted pyrrolidinyl cathinone derivatives developed in 2015, the alpha-cyclopentyl derivative was found to have around the same potency in vitro as an inhibitor of the dopamine transporter as the alpha-propyl derivative α-PVP, while the alpha-cyclohexyl derivative α-PCyP was around twice as strong.

Ethinylandrostenediol, also known as 17α-ethynyl-5-androstenediol, is a synthetic estrogen, progestogen, and androgen which was never marketed. It is the C17α ethynyl derivative of the androgen precursor and prohormone 5-androstenediol.

Fluadinazolam is a benzodiazepine derivative developed in 1973, with sedative and anxiolytic effects. It is a derivative of the never commercially marketed benzodiazepine adinazolam and has similarly been sold as a designer drug.
















