
The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.
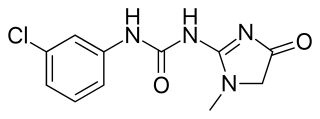
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5, and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.

Metabotropic glutamate receptor 2 (mGluR2) is a protein that, in humans, is encoded by the GRM2 gene. mGluR2 is a G protein-coupled receptor (GPCR) that couples with the Gi alpha subunit. The receptor functions as an autoreceptor for glutamate, that upon activation, inhibits the emptying of vesicular contents at the presynaptic terminal of glutamatergic neurons.

Metabotropic glutamate receptor 3 (mGluR3) is an inhibitory Gi/G0-coupled G-protein coupled receptor (GPCR) generally localized to presynaptic sites of neurons in classical circuits. However, in higher cortical circuits in primates, mGluR3 are localized post-synaptically, where they strengthen rather than weaken synaptic connectivity. In humans, mGluR3 is encoded by the GRM3 gene. Deficits in mGluR3 signaling have been linked to impaired cognition in humans, and to increased risk of schizophrenia, consistent with their expanding role in cortical evolution.

Metabotropic receptor 4 is a protein that in humans is encoded by the GRM4 gene.

Metabotropic glutamate receptor 5 is an excitatory Gq-coupled G protein-coupled receptor predominantly expressed on the postsynaptic sites of neurons. In humans, it is encoded by the GRM5 gene.

Metabotropic glutamate receptor 7 is a protein that in humans is encoded by the GRM7 gene.
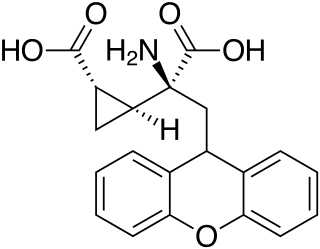
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).

Biphenylindanone A is a research agent which acts as a potent and selective positive allosteric modulator for the group II metabotropic glutamate receptor subtype mGluR2.

3-( ethynyl)pyridine (MTEP) is a research drug that was developed by Merck & Co. as a selective allosteric antagonist of the metabotropic glutamate receptor subtype mGluR5. Identified through structure-activity relationship studies on an older mGluR5 antagonist MPEP, MTEP has subsequently itself acted as a lead compound for newer and even more improved drugs.

LY-344,545 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as an antagonist for the metabotropic glutamate receptor subtype mGluR5. It is an epimer of another metabotropic glutamate receptor antagonist, the mGluR2/3-selective LY-341,495.

Pomaglumetad (LY-404,039) is an amino acid analog drug that acts as a highly selective agonist for the metabotropic glutamate receptor group II subtypes mGluR2 and mGluR3. Pharmacological research has focused on its potential antipsychotic and anxiolytic effects. Pomaglumetad is intended as a treatment for schizophrenia and other psychotic and anxiety disorders by modulating glutamatergic activity and reducing presynaptic release of glutamate at synapses in limbic and forebrain areas relevant to these disorders. Human studies investigating therapeutic use of pomaglumetad have focused on the prodrug LY-2140023, a methionine amide of pomaglumetad (also called pomaglumetad methionil) since pomaglumetad exhibits low oral absorption and bioavailability in humans.

LY-307,452 is a drug used in neuroscience research, which was among the first compounds found that acts as a selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3), and was useful in early studies of this receptor family, although it has largely been replaced by newer drugs such as LY-341,495. Its molecular formula is C21H25NO4

LY-379,268 is a drug that is used in neuroscience research, which acts as a potent and selective agonist for the group II metabotropic glutamate receptors (mGluR2/3).

LY-487,379 is a drug used in scientific research that acts as a selective positive allosteric modulator for the metabotropic glutamate receptor group II subtype mGluR2. It is used to study the structure and function of this receptor subtype, and LY-487,379 along with various other mGluR2/3 agonists and positive modulators are being investigated as possible antipsychotic and anxiolytic drugs.

CECXG (3'-ethyl-LY-341,495) is a research drug which acts as a potent and selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3), with reasonable selectivity for mGluR3. While it is some five times less potent than LY-341,495 at mGluR3, it has 38x higher affinity for mGluR3 over mGluR2, making it one of the few ligands available that is able to distinguish between these two closely related receptor subtypes.

MGS-0039 is a drug that is used in neuroscientific research, which acts as a potent and selective antagonist for group II of the metabotropic glutamate receptors (mGluR2/3). It produces antidepressant and anxiolytic effects in animal studies, and has been shown to boost release of dopamine and serotonin in specific brain areas. Research has suggested this may occur through a similar mechanism as that suggested for the similarly glutamatergic drug ketamine.

CBiPES is a drug used in scientific research that acts as a selective positive allosteric modulator for the metabotropic glutamate receptor group II subtype mGluR2. It has potentially antipsychotic effects in animal models, and is used for researching the role of mGluR2 receptors in schizophrenia and related disorders.
ADX-71149, also known as JNJ-40411813 and JNJ-mGluR2-PAM, is a selective positive allosteric modulator of the mGlu2 receptor. It is being studied by Addex Therapeutics and Janssen Pharmaceuticals for the treatment of schizophrenia. It was also researched by these companies for the treatment of anxious depression, but although some efficacy was observed in clinical trials, it was not enough to warrant further development for this indication. As of 2015, ADX-71149 is in phase II clinical trials for schizophrenia.
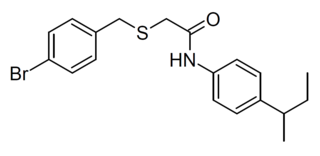
AZ-12216052 is a drug which acts as a potent and selective positive allosteric modulator of the metabotropic glutamate receptor 8, and is used for research into the role of this receptor subtype in various processes including anxiety and neuropathic pain.



















