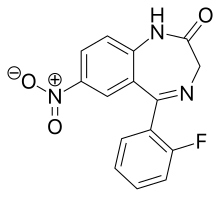
Flunitrazepam, sold under the brand name Rohypnol among others, is a benzodiazepine used to treat severe insomnia and assist with anesthesia. As with other hypnotics, flunitrazepam has been advised to be prescribed only for short-term use or by those with chronic insomnia on an occasional basis.

Adinazolam is a tranquilizer of the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. It possesses anxiolytic, anticonvulsant, sedative, and antidepressant properties. Adinazolam was developed by Jackson B. Hester, who was seeking to enhance the antidepressant properties of alprazolam, which he also developed. Adinazolam was never FDA approved and never made available to the public market; however, it has been sold as a designer drug.

Camazepam is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam. While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.

Meclonazepam ((S)-3-methylclonazepam) was discovered by a team at Hoffmann-La Roche in the 1970s and is a drug which is a benzodiazepine derivative similar in structure to clonazepam. It has sedative and anxiolytic actions like those of other benzodiazepines, and also has anti-parasitic effects against the parasitic worm Schistosoma mansoni.

L-655,708 (FG-8094) is a nootropic drug invented in 1996 by a team working for Merck, Sharp and Dohme, that was the first compound developed which acts as a subtype-selective inverse agonist at the α5 subtype of the benzodiazepine binding site on the GABAA receptor. It acts as an inverse agonist at the α1, α2, α3 and α5 subtypes, but with much higher affinity for α5, and unlike newer α5 inverse agonists such as α5IA, L-655,708 exerts its subtype selectivity purely via higher binding affinity for this receptor subtype, with its efficacy as an inverse agonist being around the same at all the subtypes it binds to.
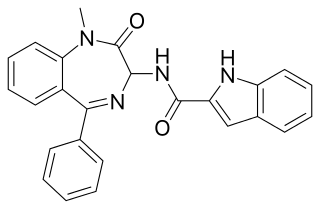
Devazepide is benzodiazepine drug, but with quite different actions from most benzodiazepines, lacking affinity for GABAA receptors and instead acting as an CCKA receptor antagonist. It increases appetite and accelerates gastric emptying, and has been suggested as a potential treatment for a variety of gastrointestinal problems including dyspepsia, gastroparesis and gastric reflux. It is also widely used in scientific research into the CCKA receptor.
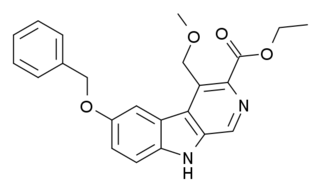
ZK-93423 is an anxiolytic drug from the β-Carboline family, closely related to abecarnil. It is a nonbenzodiazepine GABAA agonist which is not subtype selective and stimulates α1, α2, α3, and α5-subunit containing GABAA receptors equally. It has anticonvulsant, muscle relaxant and appetite stimulating properties comparable to benzodiazepine drugs. ZK-93423 has also been used as a base to develop new and improved beta-carboline derivatives and help map the binding site of the GABAA receptor.

Triflunordazepam is a drug which is a benzodiazepine derivative with high GABAA receptor affinity, and has anticonvulsant effects.

Pyrazolam (SH-I-04) is a benzodiazepine derivative originally developed by a team led by Leo Sternbach at Hoffman-La Roche in the 1970s. It has since been "rediscovered" and sold as a designer drug since 2012.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.

3-Hydroxyphenazepam is a benzodiazepine with hypnotic, sedative, anxiolytic, and anticonvulsant properties. It is an active metabolite of phenazepam, as well as the active metabolite of the benzodiazepine prodrug cinazepam. Relative to phenazepam, 3-hydroxyphenazepam has diminished myorelaxant properties, but is about equivalent in most other regards. Like other benzodiazepines, 3-hydroxyphenazepam behaves as a positive allosteric modulator of the benzodiazepine site of the GABAA receptor with an EC50 value of 10.3 nM. It has been sold as a designer drug.
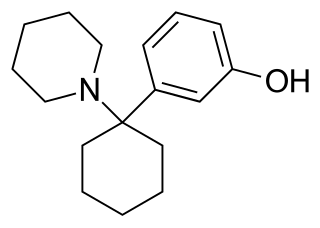
3-Hydroxyphencyclidine (3-HO-PCP) is a dissociative of the arylcyclohexylamine class related to phencyclidine (PCP) that has been sold online as a designer drug.
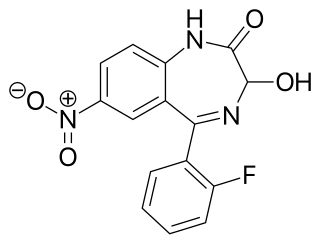
Nifoxipam is a benzodiazepine that is a minor metabolite of flunitrazepam and has been sold online as a designer drug.

Metizolam is a thienotriazolodiazepine that is the demethylated analogue of the closely related etizolam.

Nitrazolam is a triazolobenzodiazepine (TBZD) , which are benzodiazepine (BZD) derivatives, that has been sold online as a designer drug.
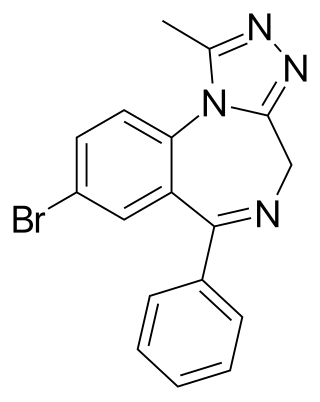
Bromazolam (XLI-268) is a triazolobenzodiazepine (TBZD) which was first synthesised in 1976, but was never marketed. It has subsequently been sold as a designer drug, first being definitively identified by the EMCDDA in Sweden in 2016. It is the bromo instead of chloro analogue of alprazolam and has similar sedative and anxiolytic effects to it and other benzodiazepines. Bromazolam is a non subtype selective agonist at the benzodiazepine site of GABAA receptors, with a binding affinity of 2.81 nM at the α1 subtype, 0.69 nM at α2 and 0.62 nM at α5. The "common" dosage range for users of bromazolam was reported to be 1–2 mg, suggesting its potency is similar to alprazolam.
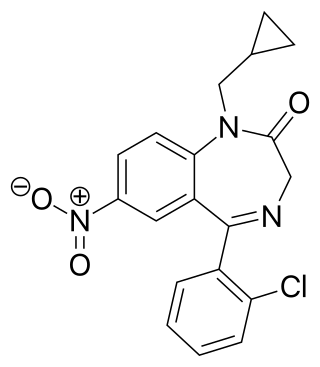
Cloniprazepam is a benzodiazepine derivative and a prodrug of clonazepam, 7-aminoclonazepam, and other metabolites.

Difludiazepam (Ro07-4065) is a benzodiazepine derivative which is the 2',6'-difluoro derivative of fludiazepam. It was invented in the 1970s but was never marketed, and has been used as a research tool to help determine the shape and function of the GABAA receptors, at which it has an IC50 of 4.1nM. Difludiazepam has subsequently been sold as a designer drug, and was first notified to the EMCDDA by Swedish authorities in 2017.

Ro07-5220 (6'-Chlorodiclazepam) is a benzodiazepine derivative with sedative, anxiolytic, anticonvulsant and muscle relaxant effects, which has been sold as a designer drug.