
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

The glycine receptor is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride currents. It is one of the most widely distributed inhibitory receptors in the central nervous system and has important roles in a variety of physiological processes, especially in mediating inhibitory neurotransmission in the spinal cord and brainstem.
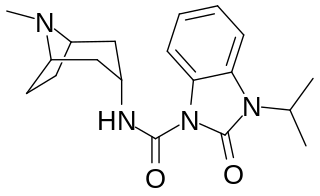
BIMU-8 is a drug which acts as a 5-HT4 receptor selective agonist. BIMU-8 was one of the first compounds of this class. The main action of BIMU-8 is to increase the rate of respiration by activating an area of the brain stem known as the pre-Botzinger complex.

NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for humans and animals; the state of anesthesia they induce is referred to as dissociative anesthesia.

Oxindole (2-indolone) is an aromatic heterocyclic organic compound with the formula C6H4CHC(O)NH. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. Oxindole is a modified indoline with a substituted carbonyl at the second position of the 5-member indoline ring. Classified as a cyclic amide, it is a pale yellow solid.

7-Hydroxymitragynine (7-OH) is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as kratom. It was first described in 1994 and is a natural product derived from the mitragynine present in the kratom leaf. 7-OH binds to opioid receptors like mitragynine, but research suggests that 7-OH binds with greater efficacy.
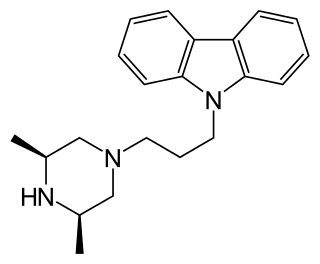
Rimcazole is an antagonist of the sigma receptor as well as a dopamine reuptake inhibitor. Sigma receptors are thought to be involved in the drug psychosis that can be induced by some drugs such as phencyclidine and cocaine, and rimcazole was originally researched as a potential antipsychotic with a different mechanism of action to traditional antipsychotic drugs. Trials proved inconclusive and rimcazole was not pursued for this application, but other sigma antagonists continue to be researched for a variety of potential applications. Rimcazole has been shown to reduce the effects of cocaine, and analogues of rimcazole have been shown to be highly effective at blocking the convulsions caused by cocaine overdose in animal models.

Rhynchophylline is an alkaloid found in certain Uncaria species (Rubiaceae), notably Uncaria rhynchophylla and Uncaria tomentosa. It also occurs in the leaves of Mitragyna speciosa (kratom) and Mitragyna tubulosa, a tree native to Thailand. Chemically, it is related to the alkaloid mitragynine.

Dotarizine is a drug used in the treatment of migraine, which acts as a calcium channel blocker, and also as an antagonist at the 5HT2A receptor, and to a lesser extent at the 5HT1A and 5HT2C receptors. The anti-migraine action is thought to be due to its action as a vasodilator, but it also has some anxiolytic effects and blocks amnesia produced by electroconvulsive shock in animals.

BIIE-0246 is a drug used in scientific research which acts as a potent and selective antagonist for the Neuropeptide Y receptor Y2. It was one of the first non-peptide Y2-selective antagonists developed, and remains among the most widely used tools for studying this receptor. It has been used to demonstrate a role for the Y2 subtype as a presynaptic autoreceptor limiting further neuropeptide Y release, as well as modulating dopamine and acetylcholine release. It has also been shown to produce several behavioural effects in animals, including reducing alcohol consumption in addicted rats and anxiolytic effects, although while selective Y2 agonists are expected to be useful as anorectics, BIIE-0246 did not appear to increase appetite when administered alone.

Nantenine is an alkaloid found in the plant Nandina domestica as well as some Corydalis species. It is an antagonist of both the α1-adrenergic receptor and the serotonin 5-HT2A receptor, and blocks both the behavioral and physiological effects of MDMA in animals.

Barakol is a compound found in the plant Senna siamea, which is used in traditional herbal medicine. It has sedative and anxiolytic effects. There are contradictory pharmacological research findings concerning the toxicity of Cassia siamea and the active ingredient Barakol. One pharmacological study has shown an hepatoxic effect of Barakol while another study did not show any toxic effect at a daily dosage intake. Further research is needed to verify whether there are toxic effects of Barakol or not.

8-OH-PBZI is a drug used in scientific research which acts as a potent and selective agonist for the dopamine D3 receptor.

Pitrazepin is a competitive GABAA and glycine receptor antagonist. It has been used to study insect and snail nervous systems in scientific research.

SR144528 is a drug that acts as a potent and highly selective CB2 receptor inverse agonist, with a Ki of 0.6 nM at CB2 and 400 nM at the related CB1 receptor. It is used in scientific research for investigating the function of the CB2 receptor, as well as for studying the effects of CB1 receptors in isolation, as few CB1 agonists that do not also show significant activity as CB2 agonists are available. It has also been found to be an inhibitor of sterol O-acyltransferase, an effect that appears to be independent from its action on CB2 receptors.
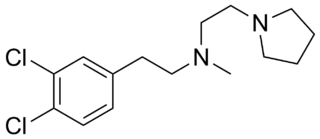
BD1008 or N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-1-pyrrolidineethanamine is a selective sigma receptor antagonist, with a reported binding affinity of Ki = 2 ± 1 nM for the sigma-1 receptor and 4 times selectivity over the sigma-2 receptor.
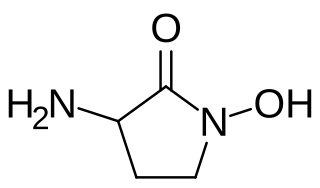
HA-966 or (±)-3-amino-1-hydroxy-pyrrolidin-2-one is a molecule used in scientific research as a glycine receptor and NMDA receptor antagonist / low efficacy partial agonist. It has neuroprotective and anticonvulsant, anxiolytic, antinociceptive and sedative / hypnotic effects in animal models. Pilot human clinical trials in the early 1960s showed that HA-966 appeared to benefit patients with tremors of extrapyramidal origin.

Mitragynine pseudoindoxyl is a rearrangement product of 7-hydroxymitragynine an active metabolite of mitragynine. It is an analgesic being more potent than morphine.

Domesticine is an α1D-adrenergic receptor antagonist. The compound belongs to the group of Aporphine alkaloids.
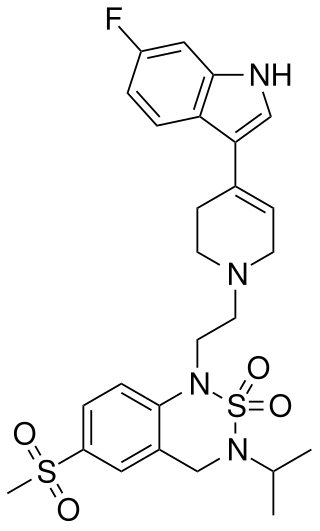
LY-393558 is a potent serotonin reuptake inhibitor and antagonist of the 5-HT1B, 5-HT1D, and 5-HT2A receptors. LY-393558 was also found to reduce serotonin-induced vasoconstriction, indicating that it may have therapeutic potential for the treatment of pulmonary hypertension.




















