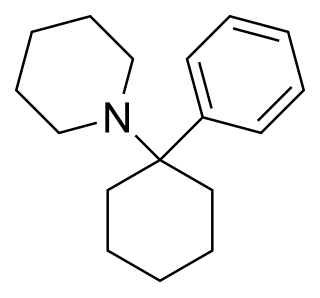
Phencyclidine or phenylcyclohexyl piperidine (PCP), also known in its use as a street drug as angel dust among other names, is a dissociative anesthetic mainly used recreationally for its significant mind-altering effects. PCP may cause hallucinations, distorted perceptions of sounds, and violent behavior. As a recreational drug, it is typically smoked, but may be taken by mouth, snorted, or injected. It may also be mixed with cannabis or tobacco.

The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and predominantly Ca2+ ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a "coincidence detector" and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.
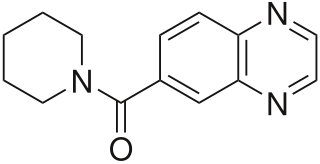
Ampakines or AMPAkines are a subgroup of AMPA receptor positive allosteric modulators with a benzamide or closely related chemical structure. They are also known as "CX compounds". Ampakines take their name from the AMPA receptor (AMPAR), a type of ionotropic glutamate receptor with which the ampakines interact and act as positive allosteric modulators (PAMs) of. Although all ampakines are AMPAR PAMs, not all AMPAR PAMs are ampakines.

Metabotropic glutamate receptor 2 (mGluR2) is a protein that, in humans, is encoded by the GRM2 gene. mGluR2 is a G protein-coupled receptor (GPCR) that couples with the Gi alpha subunit. The receptor functions as an autoreceptor for glutamate, that upon activation, inhibits the emptying of vesicular contents at the presynaptic terminal of glutamatergic neurons.

Sodium- and chloride-dependent glycine transporter 1, also known as glycine transporter 1, is a protein that in humans is encoded by the SLC6A9 gene which is promising therapeutic target for treatment of diabetes and obesity.

IDRA-21 is a positive allosteric modulator of the AMPA receptor and a benzothiadiazine derivative. It is a chiral molecule, with (+)-IDRA-21 being the active form.

LY-503430 is an AMPA receptor positive allosteric modulator developed by Eli Lilly.
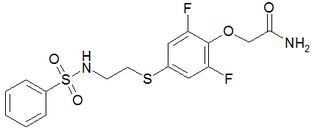
PEPA is a sulfonamide AMPA receptor positive allosteric modulator, which is up to 100 times more potent than aniracetam in vitro. It produces memory-enhancing effects in rats when administered intravenously.

LY-404187 is an AMPA receptor positive allosteric modulator which was developed by Eli Lilly and Company. It is a member of the biarylpropylsulfonamide class of AMPA receptor potentiators.
NMDA receptor modulators are a new form of antipsychotic that are in Phase II FDA studies. The first compound studied was glycine which was hypothesized by Daniel Javitt after observation that people with phencyclidine(PCP)-induced psychosis were lacking in glutamate transmission. In giving glycine to people with PCP-induced psychosis a recovery rate was noted. From there, it was hypothesized that people with psychosis from schizophrenia would benefit from increased glutamate transmission and glycine was added with strong recovery rates noted especially in the area of negative and cognitive symptoms. Glycine, however, sporadic results aside remains an adjunct antipsychotic and an unworkable compound. However, the Eli Lilly and Company study drug LY-2140023 is being studied as a primary antipsychotic and is showing strong recovery rates, especially in the area of negative and cognitive symptoms of schizophrenia. Tardive dyskinesia, diabetes and other standard complications have not been noted:
Treatment with LY2140023, like treatment with olanzapine, was safe and well-tolerated; treated patients showed statistically significant improvements in both positive and negative symptoms of schizophrenia compared to placebo. Notably, patients treated with LY-2140023 did not differ from placebo-treated patients with respect to prolactin elevation, extrapyramidal symptoms or weight gain. These data suggest that mGlu2/3 receptor agonists have antipsychotic properties and may provide a new alternative for the treatment of schizophrenia.

Perampanel, sold under the brand name Fycompa, is an anti-epileptic medication developed by Eisai Co. that is used in addition to other drugs to treat partial seizures and generalized tonic-clonic seizures for people older than twelve years. It was first approved in 2012, and as of 2016, its optimal role in the treatment of epilepsy relative to other drugs was not clear. It was the first antiepileptic drug in the class of selective non-competitive antagonist of AMPA receptors.
In pharmacology and biochemistry, allosteric modulators are a group of substances that bind to a receptor to change that receptor's response to stimuli. Some of them, like benzodiazepines or alcohol, function as psychoactive drugs. The site that an allosteric modulator binds to is not the same one to which an endogenous agonist of the receptor would bind. Modulators and agonists can both be called receptor ligands.

Sunifiram is an experimental drug which has antiamnesic effects in animal studies and with significantly higher potency than piracetam. Sunifiram is a molecular simplification of unifiram (DM-232). Another analogue is sapunifiram (MN-19). As of 2016, sunifiram had not been subjected to toxicology testing, nor to any human clinical trials, and is not approved for use anywhere in the world.

ORG-26576 is an ampakine originally developed by Cortex Pharmaceuticals and then licensed to Organon International for development. In animal studies it has been shown to effectively potentiate AMPA receptor function, leading to increased BDNF release and enhanced neuronal differentiation and survival, as well as producing nootropic effects in standardised assays. Development as an antidepressant has been halted due to a failed Phase II trial for major depressive disorder.

Mibampator is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which was under development by Eli Lilly for the treatment of agitation/aggression in Alzheimer's disease but was never marketed. It reached phase II clinical trials prior to the discontinuation of its development.

Tulrampator is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which is under development by RespireRx Pharmaceuticals and Servier for the treatment of major depressive disorder, Alzheimer's disease, dementia, and mild cognitive impairment. Tulrampator was in phase II clinical trial for depression, but failed to show superiority over placebo. There are also phase II clinical trials for Alzheimer's disease and phase I trials for dementia and mild cognitive impairment.

AMPA receptor positive allosteric modulators are positive allosteric modulators (PAMs) of the AMPA receptor (AMPR), a type of ionotropic glutamate receptor which mediates most fast synaptic neurotransmission in the central nervous system.
A GABAA receptor negative allosteric modulator is a negative allosteric modulator (NAM), or inhibitor, of the GABAA receptor, a ligand-gated ion channel of the major inhibitory neurotransmitter γ-aminobutyric acid (GABA). They are closely related and similar to GABAA receptor antagonists. The effects of GABAA receptor NAMs are functionally the opposite of those of GABAA receptor positive allosteric modulators (PAMs) like the benzodiazepines, barbiturates, and ethanol (alcohol). Non-selective GABAA receptor NAMs can produce a variety of effects including convulsions, neurotoxicity, and anxiety, among others.
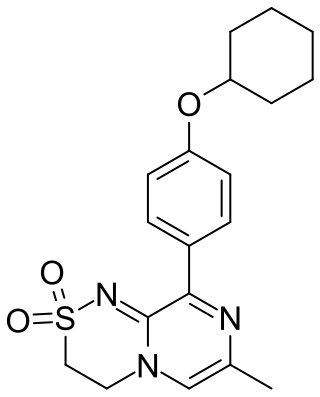
Osavampator is an experimental drug being investigated as a treatment for treatment-resistant depression. It is being developed by Takeda Pharmaceuticals.















