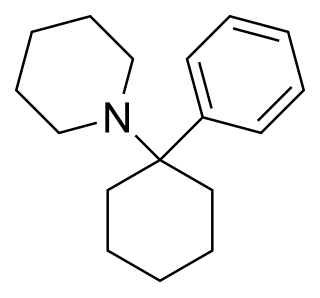
Phencyclidine or phenylcyclohexyl piperidine (PCP), also known in its use as a street drug as angel dust among other names, is a dissociative anesthetic mainly used recreationally for its significant mind-altering effects. PCP may cause hallucinations, distorted perceptions of sounds, and violent behavior. As a recreational drug, it is typically smoked, but may be taken by mouth, snorted, or injected. It may also be mixed with cannabis or tobacco.

The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a “coincidence detector” and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.

Hyperalgesia is an abnormally increased sensitivity to pain, which may be caused by damage to nociceptors or peripheral nerves and can cause hypersensitivity to stimulus. Prostaglandins E and F are largely responsible for sensitizing the nociceptors. Temporary increased sensitivity to pain also occurs as part of sickness behavior, the evolved response to infection.
Neuropathic pain is pain caused by damage or disease affecting the somatosensory system. Neuropathic pain may be associated with abnormal sensations called dysesthesia or pain from normally non-painful stimuli (allodynia). It may have continuous and/or episodic (paroxysmal) components. The latter resemble stabbings or electric shocks. Common qualities include burning or coldness, "pins and needles" sensations, numbness and itching.

Dizocilpine (INN), also known as MK-801, is a pore blocker of the N-Methyl-D-aspartate (NMDA) receptor, a glutamate receptor, discovered by a team at Merck in 1982. Glutamate is the brain's primary excitatory neurotransmitter. The channel is normally blocked with a magnesium ion and requires depolarization of the neuron to remove the magnesium and allow the glutamate to open the channel, causing an influx of calcium, which then leads to subsequent depolarization. Dizocilpine binds inside the ion channel of the receptor at several of PCP's binding sites thus preventing the flow of ions, including calcium (Ca2+), through the channel. Dizocilpine blocks NMDA receptors in a use- and voltage-dependent manner, since the channel must open for the drug to bind inside it. The drug acts as a potent anti-convulsant and probably has dissociative anesthetic properties, but it is not used clinically for this purpose because of the discovery of brain lesions, called Olney's lesions (see below), in laboratory rats. Dizocilpine is also associated with a number of negative side effects, including cognitive disruption and psychotic-spectrum reactions. It inhibits the induction of long term potentiation and has been found to impair the acquisition of difficult, but not easy, learning tasks in rats and primates. Because of these effects of dizocilpine, the NMDA receptor pore blocker ketamine is used instead as a dissociative anesthetic in human medical procedures. While ketamine may also trigger temporary psychosis in certain individuals, its short half-life and lower potency make it a much safer clinical option. However, dizocilpine is the most frequently used uncompetitive NMDA receptor antagonist in animal models to mimic psychosis for experimental purposes.

Acamprosate, sold under the brand name Campral, is a medication used along with counseling to treat alcohol use disorder.

Levorphanol is an opioid medication used to treat moderate to severe pain. It is the levorotatory enantiomer of the compound racemorphan. Its dextrorotatory counterpart is dextrorphan.

NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for animals and humans; the state of anesthesia they induce is referred to as dissociative anesthesia.

Midafotel is a potent, competitive antagonist at the NMDA receptor. It was originally designed as a potential therapy for excitotoxicity, epilepsy or neuropathic pain. It looked very promising in in vitro trials proving to be a potent competitive antagonist at the NMDA without affecting other receptors. Research continued through to in vivo cat studies where it proved to limit damage after occluding the middle cerebral artery, leading to ischaemia. It also blocked photosensitive epilepsies in baboons.

Selfotel (CGS-19755) is a drug which acts as a competitive NMDA antagonist, directly competing with glutamate for binding to the receptor. Initial studies showed it to have anticonvulsant, anxiolytic, analgesic and neuroprotective effects, and it was originally researched for the treatment of stroke, but subsequent animal and human studies showed phencyclidine-like effects, as well as limited efficacy and evidence for possible neurotoxicity under some conditions, and so clinical development was ultimately discontinued.

Remacemide is a drug which acts as a low-affinity NMDA antagonist with sodium channel blocking properties. It has been studied for the treatment of acute ischemic stroke, epilepsy, Huntington's disease, and Parkinson's disease.

CGP-37849 is a competitive antagonist at the NMDA receptor. It is a potent, orally active anticonvulsant in animal models, and was researched for the treatment of epilepsy. It also has neuroprotective activity and shows antidepressant and anxiolytic effects.

PEAQX is a competitive antagonist at the NMDA receptor. Although originally described as 100-fold selective for GluN1/GluN2A receptors vs. GluN1/GluN2B receptors, more detailed studies of the Ki of PEAQX revealed it only shows a 5 fold difference in affinity for GluN1/GluN2A vs. GluN1/GluN2B receptors. It is also a potent anticonvulsant in animal tests.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

Traxoprodil is a drug developed by Pfizer which acts as an NMDA antagonist, selective for the NR2B subunit. It has neuroprotective, analgesic, and anti-Parkinsonian effects in animal studies. Traxoprodil has been researched in humans as a potential treatment to lessen the damage to the brain after stroke, but results from clinical trials showed only modest benefit. The drug was found to cause EKG abnormalities and its clinical development was stopped. More recent animal studies have suggested traxoprodil may exhibit rapid-acting antidepressant effects similar to those of ketamine, although there is some evidence for similar psychoactive side effects and abuse potential at higher doses, which might limit clinical acceptance of traxoprodil for this application.
Conantokins are a small family of helical peptides that are derived from the venom of predatory marine snails of the genus Conus. Conantokins act as potent and specific antagonists of the N-methyl-D-aspartate receptor (NMDAR). They are the only naturally-derived peptides to do so. The subtypes of conantokins exhibit a surprising variability of selectivity across the NMDAR subunits, and are therefore uniquely useful in developing subunit-specific pharmacological probes.

HA-966 or (±) 3-Amino-1-hydroxy-pyrrolidin-2-one is a molecule used in scientific research as a glycine receptor and NMDA receptor antagonist / low efficacy partial agonist. It has neuroprotective and anticonvulsant, anxiolytic, antinociceptive and sedative / hypnotic effects in animal models. Pilot human clinical trials in the early 1960s showed that HA-966 appeared to benefit patients with tremors of extrapyramidal origin.

LY-235959 is a competitive antagonist at the NMDA receptor. It has analgesic and neuroprotective effects and causes hypothermia in animal models, as well as reducing the development of tolerance to morphine and altering the reinforcing effects of cocaine.
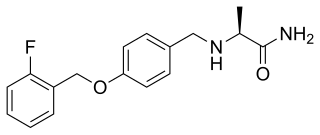
Ralfinamide (INN) is a multimodal drug which is under investigation by Newron Pharmaceuticals for the treatment of neuropathic pain and other pain conditions such as post-operative dental pain.

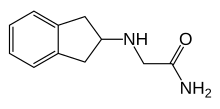
L-4-Chlorokynurenine is an orally active small molecule prodrug of 7-chlorokynurenic acid, a NMDA receptor antagonist. It was investigated as a potential rapid-acting antidepressant.