Streptomyces is the largest genus of Actinobacteria and the type genus of the family Streptomycetaceae. Over 500 species of Streptomyces bacteria have been described. As with the other Actinobacteria, streptomycetes are gram-positive, and have genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin.
Streptomyces achromogenes is a species of gram-positive bacterium that belongs in the genus Streptomyces. S. achromogenes can be grown at 28 °C in a medium of yeast and malt extract with glucose.

Hitachimycin, also known as stubomycin, is a cyclic polypeptide produced by Streptomyces that acts as an antibiotic. It exhibits cytotoxic activity against mammalian cells, Gram-positive bacteria, yeast, and fungi, as well as hemolytic activity; this is mediated by changes at the cell membrane and subsequent lysis. Owing to its cytotoxic activity against mammalian cells and tumors, it was first proposed as an antitumor antibiotic.
In enzymology, a gentamicin 2"-nucleotidyltransferase is an enzyme that catalyzes the chemical reaction

ψ-Tectorigenin is an O-methylated isoflavone, a type of flavonoid. It can be isolated from Belamcanda chinensis, Dalbergia sissoo. It can also be isolated from the bacterium Nocardiopsis sp, and from the mold Stemphilium sp. No. 644.

Callystatin A is a polyketide natural product from the leptomycin family of secondary metabolites. It was first isolated in 1997 from the marine sponge Callyspongia truncata which was collected from the Goto Islands in the Nagasaki Prefecture of Japan by the Kobayashi group. Since then its absolute configuration has been elucidated and callystatin A was discovered to have anti-fungal and anti-tumor activities with extreme potency against the human epidermoid carcinoma KB cells (IG50 = 10 pg/ml) and the mouse lymphocytic leukemia Ll210 cells (IG50 = 20 pg/ml).
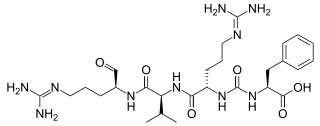
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain. It was discovered in 1972 and was the first natural peptide found that contained an ureylene group.

Statine is a gamma amino acid that occurs twice in the sequence of pepstatin, a protease inhibitor that is active against pepsin and other acid proteases. It is thought to be responsible for the inhibitory activity of pepstatin because it mimics the tetrahedral transition state of peptide catalysis.
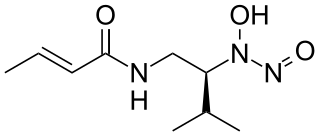
Dopastin is a chemical compound produced by the bacteria Pseudomonas No. BAC-125. It was first isolated and characterized in 1972. It is an inhibitor of the enzyme dopamine β-hydroxylase.
Streptomyces nodosus is a bacterial species in the genus Streptomyces.
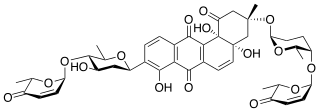
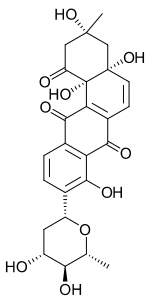
Saquayamycins are "aquayamycin-type" antibiotics isolated from Streptomyces nodosus.
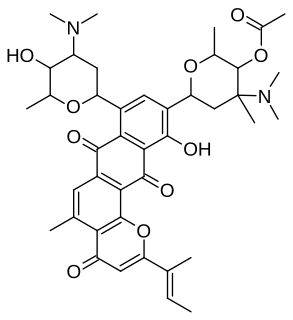
Neopluramycin is an antibiotic that inhibits nucleic acid synthesis. It has been isolated from the cultured broth of a strain of Streptomyces pluricolorescens as orange crystals, and analytical data and molecular weight determination are consistent with the empirical formula C
41H
50N
2O
10.
Naphthomycins are a group of closely related antimicrobial chemical compounds isolated from Streptomyces. They are considered a subclass of ansamycins.
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of actinobacteria, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.
Streptomyces verticillus is a species of Gram-positive bacteria in the genus Streptomyces. Whilst screening fermentation broths of this species for bioactivity in the early 1960s, Hamao Umezawa and colleagues at the Institute of Microbial Chemistry in Tokyo identified a family of glycopeptide antitumor antibiotics called the bleomycins. Examples of the bleomycins in clinical use include bleomycin A2 (also known as bleomycin) and bleomycin A5 (also known as pingyangmycin). Both are used to treat lymphomas (e.g. Hodgkin's lymphoma), head and neck cancer, and testicular cancer.
Streptomyces coeruleorubidus is a bacterium species from the genus of Streptomyces which has been isolated from marine sediment. Streptomyces coeruleorubidus produces the following medications: pacidamycin 1, baumycin B1, baumycin B2, baumycin C1, feudomycin A, feudomycin B, feudomycin C, ficellomycin, feudomycinone A, and rubomycin.
Streptomyces kasugaensis is a bacterium species from the genus of Streptomyces which has been isolated from soil from the city Nara in Japan. Streptomyces kasugaensis produces kasugamycin and thiolutin.
Streptomyces nitrosporeus is a bacterium species from the genus of Streptomyces which has been isolated from garden soil in Japan. Streptomyces nitrosporeus produces Benzastatin E, Benzastatin F, Benzastatin G Nitrosporeusine A and Nitrosporeusine B and the antibiotics nitrosporin and virantomycin and the inhibitor of angiotensin-converting enzyme foroxymithine. Streptomyces nitrosporeus can degrade cellulose.

Angucyclines are antibiotics isolated from Streptomyces species, which are used in chemotherapy as cytostatics against various types of cancer. The angucyclines include for example aquayamycin, the landomycins, moromycins, saquayamycins, urdamycins, and vineomycins.
Karen Bush is an American biochemist. She is a biology professor at Indiana University and the interim director of the university's biotechnology program. Bush conducts research focusing on bacterial resistance mechanisms to beta-lactam antibiotics.