
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders.

Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex, touching on diverse functions including mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction.

Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 (niacin). It is encoded by the codon UGG.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, social phobia, chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. Off-label uses include treatments for attention-deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), and migraine prevention. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

5-Hydroxytryptophan (5-HTP), also known as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin.
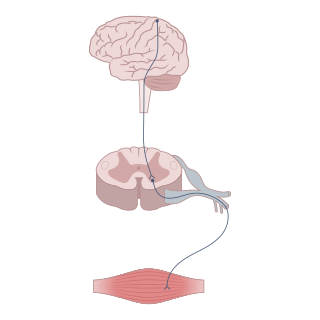
Neuromodulation is the physiological process by which a given neuron uses one or more chemicals to regulate diverse populations of neurons. Neuromodulators typically bind to metabotropic, G-protein coupled receptors (GPCRs) to initiate a second messenger signaling cascade that induces a broad, long-lasting signal. This modulation can last for hundreds of milliseconds to several minutes. Some of the effects of neuromodulators include altering intrinsic firing activity, increasing or decreasing voltage-dependent currents, altering synaptic efficacy, increasing bursting activity and reconfiguring synaptic connectivity.

Tyrosine hydroxylase or tyrosine 3-monooxygenase is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). It does so using molecular oxygen (O2), as well as iron (Fe2+) and tetrahydrobiopterin as cofactors. L-DOPA is a precursor for dopamine, which, in turn, is a precursor for the important neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). Tyrosine hydroxylase catalyzes the rate limiting step in this synthesis of catecholamines. In humans, tyrosine hydroxylase is encoded by the TH gene, and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla. Tyrosine hydroxylase, phenylalanine hydroxylase and tryptophan hydroxylase together make up the family of aromatic amino acid hydroxylases (AAAHs).

Tryptophan hydroxylase (TPH) is an enzyme (EC 1.14.16.4) involved in the synthesis of the monoamine neurotransmitter serotonin. Tyrosine hydroxylase, phenylalanine hydroxylase, and tryptophan hydroxylase together constitute the family of biopterin-dependent aromatic amino acid hydroxylases. TPH catalyzes the following chemical reaction
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission. The naturally-occurring and potent SNDRI cocaine is widely used recreationally and often illegally for the euphoric effects it produces.

In enzymology, a kynurenine 3-monooxygenase (EC 1.14.13.9) is an enzyme that catalyzes the chemical reaction

Tryptophan hydroxylase 2 (TPH2) is an isozyme of tryptophan hydroxylase found in vertebrates. In humans, TPH2 is primarily expressed in the serotonergic neurons of the brain, with the highest expression in the raphe nucleus of the midbrain. Until the discovery of TPH2 in 2003, serotonin levels in the central nervous system were believed to be regulated by serotonin synthesis in peripheral tissues, in which tryptophan hydroxylase is the dominant form.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

Quinolinic acid, also known as pyridine-2,3-dicarboxylic acid, is a dicarboxylic acid with a pyridine backbone. It is a colorless solid. It is the biosynthetic precursor to niacin.

Fenclonine, also known as para-chlorophenylalanine (PCPA), acts as a selective and irreversible inhibitor of tryptophan hydroxylase, which is a rate-limiting enzyme in the biosynthesis of serotonin.
Management of depression is the treatment of depression that may involve a number of different therapies: medications, behavior therapy, psychotherapy, and medical devices.
Scientific studies have found that different brain areas show altered activity in humans with major depressive disorder (MDD), and this has encouraged advocates of various theories that seek to identify a biochemical origin of the disease, as opposed to theories that emphasize psychological or situational causes. Factors spanning these causative groups include nutritional deficiencies in magnesium, vitamin D, and tryptophan with situational origin but biological impact. Several theories concerning the biologically based cause of depression have been suggested over the years, including theories revolving around monoamine neurotransmitters, neuroplasticity, neurogenesis, inflammation and the circadian rhythm. Physical illnesses, including hypothyroidism and mitochondrial disease, can also trigger depressive symptoms.

ST-1936 (2-methyl-5-chloro-N,N-dimethyltryptamine) is a tryptamine derivative which is used in scientific research. It acts as a selective 5-HT6 receptor agonist, with a Ki of 13 nM, and much weaker action at 5-HT2B and 5-HT7 subtypes. In animal studies it has been found to increase dopamine and noradrenaline mediated signalling but decreases glutamatergic transmission, and has antidepressant effects.
The pharmacology of antidepressants is not entirely clear. The earliest and probably most widely accepted scientific theory of antidepressant action is the monoamine hypothesis, which states that depression is due to an imbalance of the monoamine neurotransmitters. It was originally proposed based on the observation that certain hydrazine anti-tuberculosis agents produce antidepressant effects, which was later linked to their inhibitory effects on monoamine oxidase, the enzyme that catalyses the breakdown of the monoamine neurotransmitters. All currently marketed antidepressants have the monoamine hypothesis as their theoretical basis, with the possible exception of agomelatine which acts on a dual melatonergic-serotonergic pathway. Despite the success of the monoamine hypothesis it has a number of limitations: for one, all monoaminergic antidepressants have a delayed onset of action of at least a week; and secondly, there are a sizeable portion (>40%) of depressed patients that do not adequately respond to monoaminergic antidepressants. Further evidence to the contrary of the monoamine hypothesis are the recent findings that a single intravenous infusion with ketamine, an antagonist of the NMDA receptor — a type of glutamate receptor — produces rapid, robust and sustained antidepressant effects. Monoamine precursor depletion also fails to alter mood. To overcome these flaws with the monoamine hypothesis a number of alternative hypotheses have been proposed, including the glutamate, neurogenic, epigenetic, cortisol hypersecretion and inflammatory hypotheses. Another hypothesis that has been proposed which would explain the delay is the hypothesis that monoamines don't directly influence mood, but influence emotional perception biases.
A neurosteroidogenesis inhibitor is a drug that inhibits the production of endogenous neurosteroids. Neurosteroids include the excitatory neurosteroids pregnenolone sulfate, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEA-S), and the inhibitory neurosteroids allopregnanolone, tetrahydrodeoxycorticosterone (THDOC), and 3α-androstanediol, among others. By inhibiting the synthesis of endogenous neurosteroids, neurosteroidogenesis inhibitors have effects in the central nervous system.















