
A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.
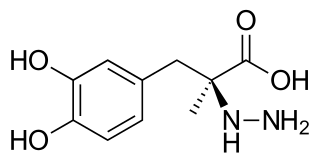
Carbidopa (Lodosyn) is a drug given to people with Parkinson's disease in order to inhibit peripheral metabolism of levodopa. This property is significant in that it allows a greater proportion of administered levodopa to cross the blood–brain barrier for central nervous system effect, instead of being peripherally metabolised into substances unable to cross said barrier.

Methyldopa, sold under the brand name Aldomet among others, is a medication used for high blood pressure. It is one of the preferred treatments for high blood pressure in pregnancy. For other types of high blood pressure including very high blood pressure resulting in symptoms other medications are typically preferred. It can be given by mouth or injection into a vein. Onset of effects is around 5 hours and they last about a day.

Alpha-methyl-p-tyrosine (AMPT) is a tyrosine hydroxylase enzyme inhibitor and is therefore a drug involved in inhibiting the catecholamine biosynthetic pathway. AMPT inhibits tyrosine hydroxylase whose enzymatic activity is normally regulated through the phosphorylation of different serine residues in regulatory domain sites. Catecholamine biosynthesis starts with dietary tyrosine, which is hydroxylated by tyrosine hydroxylase and it is hypothesized that AMPT competes with tyrosine at the tyrosine-binding site, causing inhibition of tyrosine hydroxylase.

Dopaminergic means "related to dopamine" (literally, "working on dopamine"), dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain. Dopaminergic brain pathways facilitate dopamine-related activity. For example, certain proteins such as the dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT2), and dopamine receptors can be classified as dopaminergic, and neurons that synthesize or contain dopamine and synapses with dopamine receptors in them may also be labeled as dopaminergic. Enzymes that regulate the biosynthesis or metabolism of dopamine such as aromatic L-amino acid decarboxylase or DOPA decarboxylase, monoamine oxidase (MAO), and catechol O-methyl transferase (COMT) may be referred to as dopaminergic as well. Also, any endogenous or exogenous chemical substance that acts to affect dopamine receptors or dopamine release through indirect actions (for example, on neurons that synapse onto neurons that release dopamine or express dopamine receptors) can also be said to have dopaminergic effects, two prominent examples being opioids, which enhance dopamine release indirectly in the reward pathways, and some substituted amphetamines, which enhance dopamine release directly by binding to and inhibiting VMAT2.

Phenylacetone is an organic compound with the chemical formula C6H5CH2COCH3. It is a colorless oil that is soluble in organic solvents. This substance is used in the manufacture of methamphetamine and amphetamine, where it is commonly known as P2P. Due to the illicit uses in clandestine chemistry, it was declared a schedule II controlled substance in the United States in 1980. In humans, phenylacetone occurs as a metabolite of amphetamine and methamphetamine via FMO3-mediated oxidative deamination.
Bial is a pharmaceutical company headquartered in São Mamede do Coronado, in Trofa, Porto district, Portugal. It was founded in 1924, being among the largest companies of its kind in Portugal. Its products are sold in pharmacies in more than 58 countries in 4 continents: Europe, America, Africa and Asia.
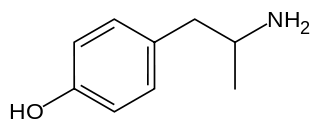
4-Hydroxyamphetamine (4HA), also known as hydroxyamfetamine, hydroxyamphetamine, oxamphetamine, norpholedrine, para-hydroxyamphetamine, and α-methyltyramine, is a drug that stimulates the sympathetic nervous system.

Tyrosine hydroxylase or tyrosine 3-monooxygenase is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). It does so using molecular oxygen (O2), as well as iron (Fe2+) and tetrahydrobiopterin as cofactors. L-DOPA is a precursor for dopamine, which, in turn, is a precursor for the important neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). Tyrosine hydroxylase catalyzes the rate limiting step in this synthesis of catecholamines. In humans, tyrosine hydroxylase is encoded by the TH gene, and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla. Tyrosine hydroxylase, phenylalanine hydroxylase and tryptophan hydroxylase together make up the family of aromatic amino acid hydroxylases (AAAHs).

Bulbocapnine is an alkaloid found in Corydalis and Dicentra, genera of the plant family Fumariaceae which have caused the fatal poisoning of sheep and cattle. It has been shown to act as an acetylcholinesterase inhibitor, and inhibits biosynthesis of dopamine via inhibition of the enzyme tyrosine hydroxylase. Like apomorphine, it is reported to be an inhibitor of amyloid beta protein (Aβ) fiber formation, whose presence is a hallmark of Alzheimer's disease (AD). Bulbocapnine is thus a potential therapeutic under the amyloid hypothesis. According to the Dorlands Medical Dictionary, it "inhibits the reflex and motor activities of striated muscle. It has been used in the treatment of muscular tremors and vestibular nystagmus".

The nuclear receptor 4A2 (NR4A2) also known as nuclear receptor related 1 protein (NURR1) is a protein that in humans is encoded by the NR4A2 gene. NR4A2 is a member of the nuclear receptor family of intracellular transcription factors.

(–)-2β-Carboisopropoxy-3β-(4-iodophenyl)tropane is a stimulant drug used in scientific research, which was developed in the early 1990s. RTI-121 is a phenyltropane based, highly selective dopamine reuptake inhibitor and is derived from methylecgonidine. RTI-121 is a potent and long-lasting stimulant, producing stimulant effects for more than 10 hours after a single dose in mice which would limit its potential uses in humans, as it might have significant abuse potential if used outside a medical setting. However RTI-121 occupies the dopamine transporter more slowly than cocaine, and so might have lower abuse potential than cocaine itself.

Dopamine beta-hydroxylase (DBH), also known as dopamine beta-monooxygenase, is an enzyme that in humans is encoded by the DBH gene. Dopamine beta-hydroxylase catalyzes the conversion of dopamine to norepinephrine.

Fusaric acid is a picolinic acid derivative and an antibiotic first isolated from the fungus Fusarium heterosporium.

Nepicastat is an inhibitor of dopamine beta-hydroxylase, an enzyme that catalyzes the conversion of dopamine to norepinephrine.

Dopamine beta (β)-hydroxylase deficiency is a condition involving inadequate dopamine beta-hydroxylase. It is characterized by increased amounts of serum dopamine and the absence of norepinephrine (NE) and epinephrine. Dopamine is released, as a false neurotransmitter, in place of norepinephrine. Other names for norepinephrine include noradrenaline (NA) and noradrenalin. This condition is also sometimes referred to as "norepinephrine deficiency". Researchers of disorders such as schizophrenia are interested in studying this disorder, as patients with these specific diseases can have an increase in the amount of dopamine in their system and yet do not show other symptoms of DβH deficiency.
Dopastin is a chemical compound produced by the bacteria Pseudomonas No. BAC-125. It was first isolated and characterized in 1972. It is an inhibitor of the enzyme dopamine β-hydroxylase.

Bupicomide is a chemical compound created and manufactured by Lanospharma Laboratories Company, Ltd. It is used experimentally as a beta blocker and clinically as a strong vasodilator with the noted side effects of reduced systolic, diastolic and mean arterial pressure.

p-Hydroxynorephedrine (PHN), or 4-hydroxynorephedrine, is the para-hydroxy analog of norephedrine and an active sympathomimetic metabolite of amphetamine in humans. When it occurs as a metabolite of amphetamine, it is produced from both p-hydroxyamphetamine and norephedrine.

4-Hydroxyphenylacetone is the para-hydroxy analog of phenylacetone, an inactive metabolite of amphetamine in humans. When it occurs as a metabolite of amphetamine, it is produced directly from the inactive metabolite phenylacetone.



















