Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex, touching on diverse functions including mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction.

Serotonin syndrome (SS) is a group of symptoms that may occur with the use of certain serotonergic medications or drugs. The symptoms can range from mild to severe, and are potentially fatal. Symptoms in mild cases include high blood pressure and a fast heart rate; usually without a fever. Symptoms in moderate cases include high body temperature, agitation, increased reflexes, tremor, sweating, dilated pupils, and diarrhea. In severe cases, body temperature can increase to greater than 41.1 °C (106.0 °F). Complications may include seizures and extensive muscle breakdown.

Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 (niacin). It is encoded by the codon UGG.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, social phobia, chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. Off-label uses include treatments for attention-deficit hyperactivity disorder (ADHD), and obsessive–compulsive disorder (OCD). SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.

5-Hydroxytryptophan (5-HTP), used medically as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin.

Enterochromaffin (EC) cells are a type of enteroendocrine cell, and neuroendocrine cell. They reside alongside the epithelium lining the lumen of the digestive tract and play a crucial role in gastrointestinal regulation, particularly intestinal motility and secretion. They were discovered by Nikolai Kulchitsky.

α-Methyl-p-tyrosine (AMPT), or simply α-methyltyrosine, also known in its chiral 2-(S) form as metirosine, is a tyrosine hydroxylase enzyme inhibitor and is therefore a drug involved in inhibiting the catecholamine biosynthetic pathway. AMPT inhibits tyrosine hydroxylase whose enzymatic activity is normally regulated through the phosphorylation of different serine residues in regulatory domain sites. Catecholamine biosynthesis starts with dietary tyrosine, which is hydroxylated by tyrosine hydroxylase and it is hypothesized that AMPT competes with tyrosine at the tyrosine-binding site, causing inhibition of tyrosine hydroxylase.
Carcinoid syndrome is a paraneoplastic syndrome comprising the signs and symptoms that occur secondary to neuroendocrine tumors. The syndrome is caused by neuroendocrine tumors most often found in the gut releasing biologically active substances into the blood causing symptoms such as flushing and diarrhea, and less frequently, heart failure, vomiting and bronchoconstriction.

The median raphe nucleus(MRN), also known as the superior central nucleus, is a nucleus in the brainstem composed of polygonal, fusiform, and piriform neurons, which exists rostral to the pontine raphe nucleus. The median raphe nucleus is one of several raphe nuclei that lies on the brainstem midline. It is one of two nuclei that are situated more superior to the others. The second of these nuclei is the dorsal raphe nucleus (DRN). The MRN extends from the lower part of the dorsal raphe nucleus to an approximate position at the decussation of the superior cerebellar peduncle.
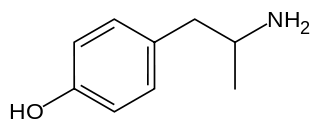
Hydroxyamphetamine, also known as 4-hydroxyamphetamine or norpholedrine and sold under the brand names Paredrine and Paremyd among others, is a sympathomimetic medication used in eye drops to dilate the pupil for eye examinations.

Tyrosine hydroxylase or tyrosine 3-monooxygenase is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). It does so using molecular oxygen (O2), as well as iron (Fe2+) and tetrahydrobiopterin as cofactors. L-DOPA is a precursor for dopamine, which, in turn, is a precursor for the important neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). Tyrosine hydroxylase catalyzes the rate limiting step in this synthesis of catecholamines. In humans, tyrosine hydroxylase is encoded by the TH gene, and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla. Tyrosine hydroxylase, phenylalanine hydroxylase and tryptophan hydroxylase together make up the family of aromatic amino acid hydroxylases (AAAHs).

Tryptophan hydroxylase (TPH) is an enzyme (EC 1.14.16.4) involved in the synthesis of the monoamine neurotransmitter serotonin. Tyrosine hydroxylase, phenylalanine hydroxylase, and tryptophan hydroxylase together constitute the family of biopterin-dependent aromatic amino acid hydroxylases. TPH catalyzes the following chemical reaction
A serotonin antagonist, or serotonin receptor antagonist, is a drug used to inhibit the action of serotonin and serotonergic drugs at serotonin (5-HT) receptors.

Tryptophan hydroxylase 2 (TPH2) is an isozyme of tryptophan hydroxylase found in vertebrates. In humans, TPH2 is primarily expressed in the serotonergic neurons of the brain, with the highest expression in the raphe nucleus of the midbrain. Until the discovery of TPH2 in 2003, serotonin levels in the central nervous system were believed to be regulated by serotonin synthesis in peripheral tissues, in which tryptophan hydroxylase is the dominant form.

5,7-Dihydroxytryptamine (5,7-DHT) is a monoaminergic neurotoxin used in scientific research to decrease concentrations of serotonin in the brain. The mechanism behind this effect is not well understood, but it is speculated to selectively destroy serotonergic neurons, in a manner similar to the dopaminergic neurotoxicity of 6-hydroxydopamine (6-OHDA). What is known is that this compound is in fact not selective in depleting serotonin content, but also depletes norepinephrine. To selectively deplete serotonin stores, it is commonly administered in conjunction with desmethylimipramine (desipramine), which inhibits the norepinephrine transporter.

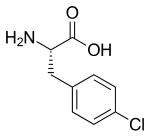
para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a serotonin–norepinephrine–dopamine releasing agent (SNDRA) and serotonergic neurotoxin of the amphetamine family. It is used in scientific research in the study of the serotonin system, as a serotonin releasing agent (SRA) at lower doses to produce serotonergic effects, and as a serotonergic neurotoxin at higher doses to produce long-lasting depletions of serotonin.
The pharmacology of antidepressants is not entirely clear.
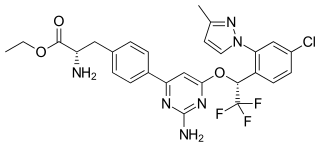
Telotristat ethyl is a prodrug of telotristat, which is an inhibitor of tryptophan hydroxylase. It is formulated as telotristat etiprate — a hippurate salt of telotristat ethyl.
Acute tryptophan depletion (ATD) is a technique used extensively to study the effect of low serotonin in the brain. This experimental approach reduces the availability of tryptophan, an amino acid which serves as the precursor to serotonin. The lack of mood-lowering effects after ATD in healthy subjects seems to contradict a direct causal relationship between acutely decreased serotonin levels and depression. However, mood-lowering effects are observed in certain vulnerable individuals. For example, a meta-analysis show that the effect size for the effects of tryptophan depletion on mood in depressed people not taking antidepressants was large (Hedge's g = −1.9 Hence, a more accurate interpretation is that tryptophan depletion studies suggest a role for 5-HT in people vulnerable to depression and in those remitted on SSRI treatment

α-Methyltryptophan is a synthetic tryptamine derivative, an artificial amino acid, and a prodrug of α-methylserotonin (αMS). It is the α-methylated derivative of tryptophan, while αMS is the α-methylated analogue of serotonin. αMTP has been suggested for potential therapeutic use in the treatment of conditions thought by some authors to be related to serotonin deficiency, such as depression. In labeled forms, αMTP is also used as a radiotracer in positron emission tomography (PET) imaging to assess serotonin synthesis and certain other processes.