A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union, Australia, and New Zealand, as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids.

HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended duration of action. HU-210 has a binding affinity of 0.061 nM at CB1 receptors compared to 40.7 nM for Δ9-THC. The binding pose of HU-210 to the CB1 receptor is similar to other synthetic cannabinoids.

N-Methyltryptamine (NMT), also known as monomethyltryptamine, is a chemical compound of the tryptamine family and a naturally occurring compound found in the human body and certain plants.

4-Methylaminorex is a stimulant drug of the 2-amino-5-aryloxazoline group that was first synthesized in 1960 by McNeil Laboratories. It is also known by its street name "U4Euh" ("Euphoria"). It is banned in many countries as a stimulant. 4-Methylaminorex has effects comparable to methamphetamine but with a longer duration.

Methylenedioxypyrovalerone is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI). It was first developed in the 1960s by a team at Boehringer Ingelheim. Its activity at the dopamine transporter is six times stronger than at the norepinephrine transporter and it is virtually inactive at the serotonin transporter. MDPV remained an obscure stimulant until around 2004 when it was reportedly sold as a designer drug. In the US, products containing MDPV and labeled as bath salts were sold as recreational drugs in gas stations, similar to the marketing for Spice and K2 as incense, until it was banned in 2011.

4'-Methyl-α-pyrrolidinopropiophenone is a stimulant drug and substituted cathinone. It is structurally very similar to α-PPP, with only one added methyl group in the para position on the phenyl ring. 4-MePPP was sold in Germany as a designer drug in the late 1990s and early 2000s, along with a number of other pyrrolidinophenone derivatives. Although it has never achieved the same international popularity as its better-known relations α-PPP and MDPV, 4-MePPP is still sometimes found as an ingredient of grey-market "bath salt" blends such as "NRG-3".

α-Pyrrolidinopentiophenone (α-PVP), also known as α-pyrrolidinovalerophenone, O-2387, β-keto-prolintane, prolintanone, or desmethylpyrovalerone, is a synthetic stimulant of the cathinone class developed in the 1960s that has been sold as a designer drug and often consumed for recreational reasons. α-PVP is chemically related to pyrovalerone and is the ketone analog of prolintane.

5-(2-Aminopropyl)indole is an indole and phenethylamine derivative with empathogenic effects. Its preparation was first reported by Albert Hofmann in 1962. It is a designer drug that has been openly sold as a recreational drug by online vendors since 2011.
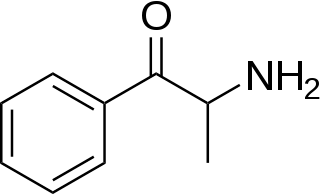
Substituted cathinones, or simply cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.

Eutylone is a stimulant and empathogenic drug of the phenethylamine, amphetamine, phenylisobutylamine, and cathinone families which was developed in the 1960s, which is classified as a designer drug. It was first reported to the EMCDDA in 2014 and became widespread internationally in 2019-2020 following bans on the related compound ephylone. It is a synthetic cathinone. In 2021, eutylone was the most common cathinone identified by the Drug Enforcement Administration in the United States.
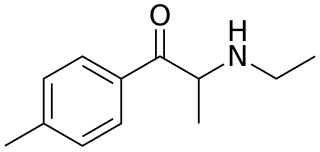
4-Methylethcathinone or 4-MEC is a chemical that bears a chemical resemblance to mephedrone. Due to its similarity to mephedrone, it is thought to be a stimulant and entactogen drug of the phenethylamine, amphetamine, and cathinone chemical classes. It has been marketed alone or in mixtures with other substituted cathinones under the name "NRG-2", although other blends such as "NRG-1" may have been more ambiguous with their ingredients.

3,4-Dimethylmethcathinone (3,4-DMMC) is a stimulant drug first reported in 2010 as a designer drug analogue of mephedrone, apparently produced in response to the banning of mephedrone, following its widespread abuse in many countries in Europe and around the world. 3,4-DMMC has been seized as a designer drug in Australia. In vitro, 3,4-DMMC was shown to be a monoamine transporter substrate that potently inhibits norepinephrine and serotonin reuptake, and to a lesser extent dopamine reuptake.

Benzedrone (4-MBC) is a designer drug which has been found since 2010 as an ingredient in a number of "bath salt" mixes sold as recreational drugs.
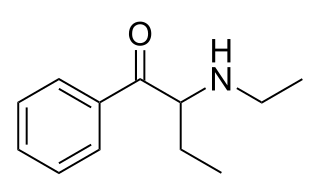
N-Ethylbuphedrone is a stimulant of the cathinone class that has been sold as a designer drug. It is the β-ketone analogue of N,alpha-diethylphenylethylamine.
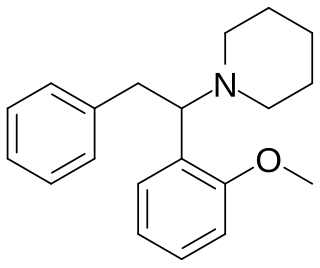
Methoxphenidine is a dissociative of the diarylethylamine class that has been sold online as a designer drug. Methoxphenidine was first reported in a 1989 patent where it was tested as a treatment for neurotoxic injury. Shortly after the 2013 UK ban on arylcyclohexylamines methoxphenidine and the related compound diphenidine became available on the gray market, where it has been encountered as a powder and in tablet form. Though diphenidine possesses higher affinity for the NMDA receptor, anecdotal reports suggest methoxphenidine has greater oral potency. Of the three isomeric anisyl-substituents methoxphenidine has affinity for the NMDA receptor that is higher than 4-MeO-diphenidine but lower than 3-MeO-diphenidine, a structure–activity relationship shared by the arylcyclohexylamines.

3-Methylmethcathinone (3-MMC), also known as metaphedrone, is a designer drug from the substituted cathinone family. 3-MMC is a monoamine transporter substrate that potently releases and inhibits the reuptake of dopamine and norepinephrine, as well as displaying moderate serotonin releasing activity.

3-Fluorophenmetrazine is a phenylmorpholine-based stimulant and fluorinated analogue of phenmetrazine that has been sold online as a designer drug.

4-Methylbuphedrone, is a stimulant drug of the cathinone class that has been sold online as a designer drug.

4-Chloromethcathinone is a stimulant drug of the cathinone class that has been sold online as a designer drug.
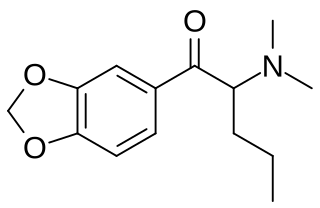
N,N-Dimethylpentylone is a substituted cathinone derivative with stimulant effects, which has been sold as a designer drug, first detected in Sweden in 2014.



















