
Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. It has a popular image as a contributor to feelings of well-being and happiness, though its actual biological function is complex and multifaceted, modulating cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction.

Tryptamine is a monoamine alkaloid. It contains an indole ring structure, and is structurally similar to the amino acid tryptophan, from which the name derives. Tryptamine is found in trace amounts in the brains of mammals and is hypothesized to play a role as a neuromodulator or neurotransmitter. Similar to other trace amines, tryptamine binds to human trace amine-associated receptor 1 (TAAR1) as an agonist.

5-Hydroxytryptophan (5-HTP), also known as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin.

Fenfluramine, formerly sold under the brand name Pondimin among others, is an appetite suppressant which was used to treat obesity and is now no longer marketed. It was used both on its own and, in combination with phentermine, as part of the anti-obesity medication Fen-Phen.

Naphthylisopropylamine (PAL-287) is an experimental drug under investigation as of 2007 for the treatment of alcohol and stimulant addiction.

The 5-HT2C receptor is a subtype of 5-HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gq/G11 and mediates excitatory neurotransmission. HTR2C denotes the human gene encoding for the receptor, that in humans is located at the X chromosome. As males have one copy of the gene and in females one of the two copies of the gene is repressed, polymorphisms at this receptor can affect the two sexes to differing extent.

The serotonin 1A receptor is a subtype of serotonin receptor that binds the neurotransmitter serotonin. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, that mediates inhibitory neurotransmission. The serotonin 1A receptor is encoded by the HTR1A gene.

5-hydroxytryptamine (serotonin) receptor 1D, also known as HTR1D, is a 5-HT receptor, but also denotes the human gene encoding it. 5-HT1D acts on the central nervous system, and affects locomotion and anxiety. It also induces vascular vasoconstriction in the brain.

5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT).

5-Hydroxytryptamine (serotonin) receptor 5A, also known as HTR5A, is a protein that in humans is encoded by the HTR5A gene.

MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons.

SB-649,915 is a serotonin reuptake inhibitor and 5-HT1A and 5-HT1B receptor antagonist which is being investigated for its antidepressant effects. Relative to the selective serotonin reuptake inhibitors (SSRIs), SB-649,915 has a faster onset of action and may also have greater clinical efficacy as well. This can be attributed to blockade of 5-HT1A and 5-HT1B autoreceptors which inhibit serotonin release.

4-Methylamphetamine is a stimulant and anorectic drug of the phenethylamine and amphetamine chemical classes.
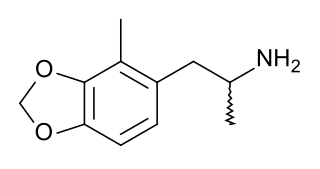
2-Methyl-3,4-methylenedioxyamphetamine (2-methyl-MDA) is an entactogen and psychedelic drug of the amphetamine class. It acts as a selective serotonin releasing agent (SSRA), with IC50 values of 93nM, 12,000nM, and 1,937nM for serotonin, dopamine, and norepinephrine efflux. 2-Methyl-MDA is more potent than MDA and 5-methyl-MDA. However, it is slightly more selective for serotonin over dopamine and norepinephrine release in comparison to 5-methyl-MDA.

3,4-Dichloroamphetamine (DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related para-chloroamphetamine, though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine N-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body.

5-Chloro-α-methyltryptamine (5-Chloro-αMT), also known as PAL-542, is a tryptamine derivative related to α-methyltryptamine (αMT) and one of only a few known specific serotonin-dopamine releasing agents (SDRAs). It has been investigated in animals as a potential treatment for cocaine dependence. The EC50 values of 5-chloro-αMT in evoking the in vitro release of serotonin (5-HT), dopamine (DA), and norepinephrine (NE) in rat synaptosomes were reported as 16 nM, 54 nM, and 3434 nM, with an NE/DA ratio of 63.6 and a DA/5-HT ratio of 3.38, indicating that it is a highly specific and well-balanced SDRA. However, 5-chloro-αMT has also been found to act as a potent full agonist of the 5-HT2A receptor, with an EC50 value of 6.27 nM and an efficacy of 105%, and almost assuredly acts as a potent agonist of other serotonin receptors as well.

Ansofaxine hydrochloride (INN) (former developmental code names LY03005, LPM570065), also known as 4-methylbenzoate desvenlafaxine hydrochloride, is a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) which is under development by Luye Pharma Group for the treatment of major depressive disorder (MDD). It is described as an SNDRI and prodrug to desvenlafaxine. However, unlike desvenlafaxine, which has in vitroIC50 values of 53 nM and 538 nM for inhibition of serotonin and norepinephrine reuptake, respectively, ansofaxine has respective in vitro IC50 values of 723 nM, 763 nM, and 491 nM for serotonin, norepinephrine, and dopamine reuptake inhibition. As of July 2018, the drug is in preregistration for MDD in the United States, the European Union, Japan, and China.

ortho-Methylphenylpiperazine (oMPP, oMePP), or 1-(2-methylphenyl)piperazine (2-MPP, 2-MePP), is a psychoactive and designer drug of the phenylpiperazine group. It acts as a serotonin–norepinephrine–dopamine releasing agent (SNDRA), with EC50 values for induction of monoamine release of 175 nM for serotonin, 39.1 nM for norepinephrine, and 296–542 nM for dopamine. As such, it has about 4.5-fold preference for induction of norepinephrine release over serotonin, and about 7.6- to 13.9-fold preference for induction of norepinephrine release over dopamine.



















