
Beta blockers, also spelled β-blockers, are a class of medications that are predominantly used to manage abnormal heart rhythms, and to protect the heart from a second heart attack after a first heart attack. They are also widely used to treat high blood pressure, although they are no longer the first choice for initial treatment of most patients.

Propranolol, sold under the brand name Inderal among others, is a medication of the beta blocker class. It is used to treat high blood pressure, a number of types of irregular heart rate, thyrotoxicosis, capillary hemangiomas, performance anxiety, and essential tremors, as well to prevent migraine headaches, and to prevent further heart problems in those with angina or previous heart attacks. It can be taken by mouth or by injection into a vein. The formulation that is taken by mouth comes in short-acting and long-acting versions. Propranolol appears in the blood after 30 minutes and has a maximum effect between 60 and 90 minutes when taken by mouth.
Antiarrhythmic agents, also known as cardiac dysrhythmia medications, are a group of pharmaceuticals that are used to suppress abnormally fast rhythms (tachycardias), such as atrial fibrillation, supraventricular tachycardia and ventricular tachycardia.

Nadolol, sold under the brand name Corgard among others, is a medication used to treat high blood pressure, heart pain, atrial fibrillation, and some inherited arrhythmic syndromes. It has also been used to prevent migraine headaches and complications of cirrhosis. It is taken orally.

Dobutamine is a medication used in the treatment of cardiogenic shock and severe heart failure. It may also be used in certain types of cardiac stress tests. It is given by IV only, as an injection into a vein or intraosseous as a continuous infusion. The amount of medication needs to be adjusted to the desired effect. Onset of effects is generally seen within 2 minutes. It has a half-life of two minutes. This drug is generally only administered short term, although it may be used for longer periods to relieve symptoms of heart failure in patients awaiting heart transplantation.
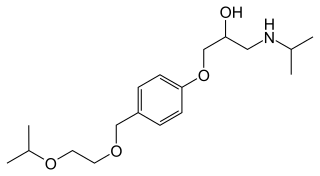
Bisoprolol, sold under the brand name Zebeta among others, is a beta blocker medication used for heart diseases. This includes tachyarrhythmias, high blood pressure, chest pain from not enough blood flow to the heart, and heart failure. It is taken by mouth.

Fenoldopam mesylate (Corlopam) is a drug and synthetic benzazepine derivative which acts as a selective D1 receptor partial agonist. Fenoldopam is used as an antihypertensive agent. It was approved by the Food and Drug Administration (FDA) in September 1997.

Mecamylamine is a non-selective, non-competitive antagonist of the nicotinic acetylcholine receptors (nAChRs) that was introduced in the 1950s as an antihypertensive drug. In the United States, it was voluntarily withdrawn from the market in 2009 but was brought to market in 2013 as Vecamyl and eventually was marketed by Turing Pharmaceuticals.
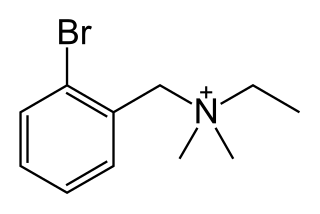
Bretylium (also bretylium tosylate) is an antiarrhythmic agent. It blocks the release of noradrenaline from nerve terminals. In effect, it decreases output from the peripheral sympathetic nervous system. It also acts by blocking K+ channels and is considered a class III antiarrhythmic. The dose is 5–10 mg/kg and side effects are high blood pressure followed by low blood pressure and ventricular ectopy.
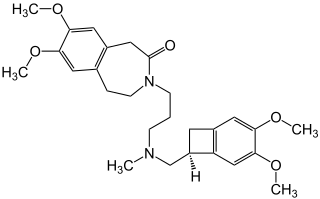
Ivabradine, sold under the brand name Procoralan among others, is a medication, which is a pacemaker current (If) inhibitor, used for the symptomatic management of heart-related chest pain and heart failure. Patients who qualify for use of Ivabradine for coronary heart failure are patients who have symptomatic heart failure, with reduced ejection volume, and heart rate at least 70 bpm, and the condition not able to be fully managed by beta blockers.

Nebivolol is a beta blocker used to treat high blood pressure and heart failure. As with other β-blockers, it is generally a less preferred treatment for high blood pressure. It may be used by itself or with other blood pressure medication. It is taken by mouth.

Alpha-adrenergic agonists are a class of sympathomimetic agents that selectively stimulates alpha adrenergic receptors. The alpha-adrenergic receptor has two subclasses α1 and α2. Alpha 2 receptors are associated with sympatholytic properties. Alpha-adrenergic agonists have the opposite function of alpha blockers. Alpha adrenoreceptor ligands mimic the action of epinephrine and norepinephrine signaling in the heart, smooth muscle and central nervous system, with norepinephrine being the highest affinity. The activation of α1 stimulates the membrane bound enzyme phospholipase C, and activation of α2 inhibits the enzyme adenylate cyclase. Inactivation of adenylate cyclase in turn leads to the inactivation of the secondary messenger cyclic adenosine monophosphate and induces smooth muscle and blood vessel constriction.

Benzoctamine is a drug that possesses sedative and anxiolytic properties. Marketed as Tacitin by Ciba-Geigy, it is different from most sedative drugs because in most clinical trials it does not produce respiratory depression, but actually stimulates the respiratory system. As a result, when compared to other sedative and anxiolytic drugs such as benzodiazepines like diazepam, it is a safer form of tranquilizing. However, when co-administered with other drugs that cause respiratory depression, like morphine, it can cause increased respiratory depression.
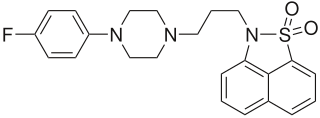
Fananserin (RP-62203) is a drug which acts as a potent antagonist at both the 5HT2A receptor, and the Dopamine D4 receptor, but without blocking other dopamine receptors such as D2. It has sedative and antipsychotic effects, and has been researched for the treatment of schizophrenia, although efficacy was less than expected and results were disappointing.
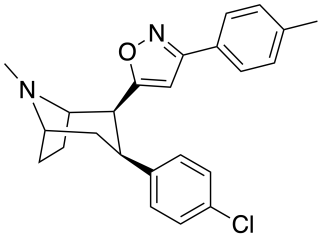
RTI(-4229)-336, is a phenyltropane derivative which acts as a potent and selective dopamine reuptake inhibitor and stimulant drug. It binds to the dopamine transporter with around 20x the affinity of cocaine, however it produces relatively mild stimulant effects, with a slow onset and long duration of action. These characteristics make it a potential candidate for treatment of cocaine addiction, as a possible substitute drug analogous to how methadone is used for treating heroin abuse. RTI-336 fully substitutes for cocaine in addicted monkeys and supports self-administration, and significantly reduces rates of cocaine use, especially when combined with SSRIs, and research is ongoing to determine whether it could be a viable substitute drug in human cocaine addicts.

F-15,599, also known as NLX-101, is a potent and selective 5-HT1A receptor full agonist. It displays functional selectivity by strongly activating 5-HT1A receptors in the postsynaptic prefrontal cortex while having little effect on somatodendritic autoreceptors in the raphe nucleus. As a result, it has been touted as a preferential postsynaptic 5-HT1A receptor agonist and has been investigated as a novel potential antidepressant.

Bromadoline (U-47931E) is an opioid analgesic selective for the μ-opioid receptor developed by the Upjohn company in the 1970s. The drug has a potency lying between that of codeine and morphine, being slightly stronger than pentazocine. Bromadoline is related to AH-7921 and U-47700.

β adrenergic receptor antagonists were initially developed in the 1960s, for the treatment of angina pectoris but are now also used for hypertension, congestive heart failure and certain arrhythmias. In the 1950s, dichloroisoproterenol (DCI) was discovered to be a β-antagonist that blocked the effects of sympathomimetic amines on bronchodilation, uterine relaxation and heart stimulation. Although DCI had no clinical utility, a change in the compound did provide a clinical candidate, pronethalol, which was introduced in 1962.
(Edward) Miles Vaughan Williams was a British cardiac pharmacologist and academic. He is best known for the Vaughan Williams classification of antidysrhythmic drugs. From 1955 to 1985, he was a Fellow of Hertford College, Oxford, and its Tutor in medicine.
Alison Marion Gurney is professor of Pharmacology at the University of Manchester. She previously held the W.C. Bowman Chair of Pharmacology at the University of Strathclyde, where she was the first female appointed to a science professorship and the first female Professor of Pharmacology in Scotland. She is known for her research into the pharmacology and physiological roles of ion channels, especially in the pulmonary circulation.


















