Metenolone, or methenolone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as metenolone acetate and metenolone enanthate. Metenolone esters are used mainly in the treatment of anemia due to bone marrow failure. Metenolone acetate is taken by mouth, while metenolone enanthate is given by injection into muscle.

Meclofenoxate is a cholinergic nootropic used as a dietary supplement. It is an ester of dimethylethanolamine (DMAE) and 4-chlorophenoxyacetic acid (pCPA).
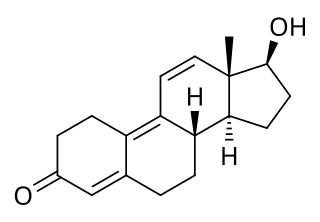
Trenbolone is an androgen and anabolic steroid (AAS) of the nandrolone group which itself was never marketed. Trenbolone ester prodrugs, including trenbolone acetate and trenbolone hexahydrobenzylcarbonate, are or have been marketed for veterinary and clinical use. Trenbolone acetate is used in veterinary medicine in livestock to increase muscle growth and appetite, while trenbolone hexahydrobenzylcarbonate was formerly used clinically in humans but is now no longer marketed. In addition, although it is not approved for clinical or veterinary use, trenbolone enanthate is sometimes sold on the black market under the nickname Trenabol.
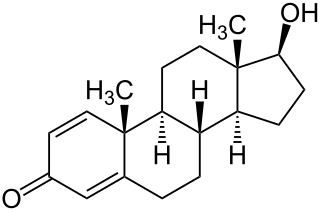
Boldenone, is a naturally occurring anabolic–androgenic steroid (AAS) and the 1(2)-dehydrogenated analogue of testosterone. Boldenone itself has never been marketed; as a pharmaceutical drug, it is used as boldenone undecylenate, the undecylenate ester.

Butorphanol is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers. Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs, cats and horses due to low bioavailability in humans.

Ethylestrenol, also known as ethyloestrenol or ethylnandrol and sold under the brand names Maxibolin and Orabolin among others, is an androgen and anabolic steroid (AAS) medication which has been used in the past for a variety of indications such as to promote weight gain and to treat anemia and osteoporosis but has been discontinued for use in humans. It is still available for veterinary use in Australia and New Zealand however. It is taken by mouth.
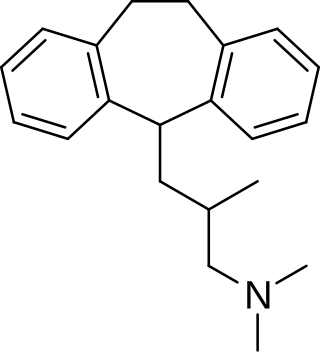
Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.

Promazine, is used as a short-term add-on treatment for psychomotor agitation. Its approved uses in people is limited, but is used as a tranquilizer in veterinary medicine. It has weak antipsychotic effects but is generally not used to treat psychoses.

Norethandrolone, sold under the brand names Nilevar and Pronabol among others, is an androgen and anabolic steroid (AAS) medication which has been used to promote muscle growth and to treat severe burns, physical trauma, and aplastic anemia but has mostly been discontinued. It is still available for use in France however. It is taken by mouth.
Pipamperone, sold under the brand name Dipiperon, is a typical antipsychotic of the butyrophenone family used in the treatment of schizophrenia and as a sleep aid for depression. It is or has been marketed under brand names including Dipiperon, Dipiperal, Piperonil, Piperonyl, and Propitan. Pipamperone was discovered at Janssen Pharmaceutica in 1961, and entered clinical trials in the United States in 1963.

Corbadrine, sold under the brand name Neo-Cobefrine and also known as levonordefrin and α-methylnorepinephrine, is a catecholamine sympathomimetic used as a topical nasal decongestant and vasoconstrictor in dentistry in the United States. It is usually used in a pre-mixed solution with local anesthetics, such as mepivacaine.

Amidephrine, or amidefrine, sold under the brand name Fentrinol among others, is a selective α1-adrenergic receptor agonist which is described as an adrenergic or sympathomimetic, vasoconstrictor, and topical nasal decongestant used to treat allergic rhinitis. It is used as the mesylate salt, which has the generic names amidefrine mesilate and amidephrine mesylate. The drug is a substituted phenethylamine derivative and is also known as 3-methylsulfonamidyl-β-hydroxy-N-methylphenethylamine. As of 2000, it remained marketed only in Austria.

Dixyrazine, also known as dixypazin (oxalate), sold under the brand names Ansiolene, Esocalm, Esucos, Metronal, and Roscal, is a typical antipsychotic of the phenothiazine group described as a neuroleptic and antihistamine. It was first introduced in Germany in 1969. It is used as a neuroleptic, anxiolytic, and antihistamine in doses between 12.5 and 75 mg a day.

Prothipendyl, also known as azapromazine or phrenotropin, is an anxiolytic, antiemetic, and antihistamine of the azaphenothiazine group which is marketed in Europe and is used to treat anxiety and agitation in psychotic syndromes. It differs from promazine only by the replacement of one carbon atom with a nitrogen atom in the tricyclic ring system. Prothipendyl is said to not possess antipsychotic effects, and in accordance, appears to be a weaker dopamine receptor antagonist than other phenothiazines.

Bolasterone, also known as 7α,17α-dimethyltestosterone, is a 17α-alkylated androgen/anabolic steroid (AAS) which is used in veterinary medicine. It has close structural similarity to testosterone, and like methyltestosterone has a methyl group at C17α in order to increase oral bioavailability. In addition, it is also 7α-methylated, similar to its 7β-methylated isomer calusterone. The medication has a low to moderate ratio of anabolic to androgenic activity, similar to that of fluoxymesterone.

Formebolone, also known as formyldienolone, as well as 2-formyl-11α-hydroxy-17α-methyl-δ1-testosterone, is an orally active anabolic-androgenic steroid (AAS) described as an anticatabolic and anabolic drug that is or has been marketed in Spain and Italy. As an AAS, it shows some anabolic activity, though it is inferior to testosterone in terms of potency, but is said to have virtually no androgenic activity. Formebolone counteracts the catabolic effects of potent glucocorticoids like dexamethasone phosphate. A close analogue, roxibolone, shows similar antiglucocorticoid activity to formebolone but, in contrast, is devoid of activity as an AAS.

Oxymesterone, also known as methandrostenediolone, as well as 4-hydroxy-17α-methyltestosterone or 17α-methylandrost-4-en-4,17β-diol-3-one, is an orally active anabolic-androgenic steroid (AAS). It was known by 1960.
Oxilofrine, sold under the brand names Carnigen and Suprifen among others, is a sympathomimetic medication which has been used as an antihypotensive agent and cough suppressant. It is taken by mouth.

Isomethadone (INN, BAN; trade name Liden; also known as isoamidone) is a synthetic opioid analgesic and antitussive related to methadone that was used formerly as a pharmaceutical drug but is now no longer marketed. Isomethadone was used as both an analgesic and antitussive. It binds to and activates both the μ- and δ-opioid receptors, with the (S)-isomer being the more potent of the two enantiomers. Isomethadone is a Schedule II controlled substance in the United States, with an ACSCN of 9226 and a 2014 aggregate manufacturing quota of 5 g. The salts in use are the hydrobromide (HBr, free base conversion ratio 0.793), hydrochloride (HCl, 0.894), and HCl monohydrate (0.850). Isomethadone is also regulated internationally as a Schedule I controlled substance under the United Nations Single Convention on Narcotic Drugs of 1961.

Mefexamide, also known as mefexadyne and mexephenamide, is a central nervous system stimulant that is no longer marketed.