Related Research Articles

Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involved in a wide range of physiological processes, including stress response, immune response, and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels, and behavior.

Fluticasone/salmeterol, sold under the brand name Advair among others, is a fixed-dose combination medication containing fluticasone propionate and salmeterol. It is used in the management of asthma and chronic obstructive pulmonary disease (COPD). It is used by inhaling the medication into the lungs.
ATC code R03Drugs for obstructive airway diseases is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup R03 is part of the anatomical group R Respiratory system.
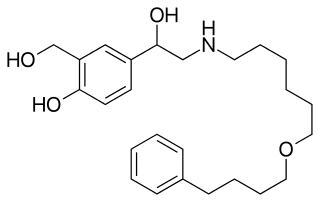
Salmeterol is a long-acting β2 adrenergic receptor agonist (LABA) used in the maintenance and prevention of asthma symptoms and maintenance of chronic obstructive pulmonary disease (COPD) symptoms. Symptoms of bronchospasm include shortness of breath, wheezing, coughing and chest tightness. It is also used to prevent breathing difficulties during exercise.

Budesonide/formoterol, sold under the brand name Symbicort among others, is a fixed-dose combination medication used in the management of asthma or chronic obstructive pulmonary disease (COPD). It contains budesonide, a steroid and formoterol, a long-acting β2-agonist (LABA). The product monograph does not support its use for sudden worsening or treatment of active bronchospasm. However, a 2020 review of the literature does support such use. It is used by breathing in the medication.

Formoterol, also known as eformoterol, is a long-acting β2 agonist (LABA) used as a bronchodilator in the management of asthma and chronic obstructive pulmonary disease (COPD). Formoterol has an extended duration of action compared to short-acting β2 agonists such as salbutamol (albuterol), which are effective for 4 h to 6 h. Formoterol has a relatively rapid onset of action compared to other LABAs, and is effective within 2-3 minutes. The 2022 Global Initiative for Asthma report recommends a combination formoterol/inhaled corticosteroid inhaler as both a preventer and reliever treatment for asthma in adults. In children, a short-acting β2 adrenergic agonist is still recommended.

Glycopyrronium bromide is a medication of the muscarinic anticholinergic group. It does not cross the blood–brain barrier and consequently has few to no central effects. It is given by mouth, via intravenous injection, on the skin, and via inhalation. It is a synthetic quaternary ammonium compound. The cation, which is the active moiety, is called glycopyrronium (INN) or glycopyrrolate (USAN).
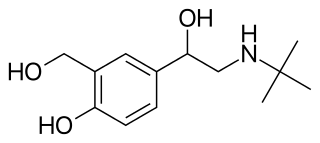
Beta2-adrenergic agonists, also known as adrenergic β2 receptor agonists, are a class of drugs that act on the β2 adrenergic receptor. Like other β adrenergic agonists, they cause smooth muscle relaxation. β2 adrenergic agonists' effects on smooth muscle cause dilation of bronchial passages, vasodilation in muscle and liver, relaxation of uterine muscle, and release of insulin. They are primarily used to treat asthma and other pulmonary disorders. Bronchodilators are considered an important treatment regime for chronic obstructive pulmonary disease (COPD) and are usually used in combination with short acting medications and long acting medications in a combined inhaler.

Long-acting β adrenoceptor agonists (LABAs) are beta-adrenergic agonists usually prescribed for moderate-to-severe persistent asthma and chronic obstructive pulmonary disease (COPD).

Mometasone, also known as mometasone y 3 s, is a steroid medication used to treat certain skin conditions, hay fever, and asthma. Specifically it is used to prevent rather than treat asthma attacks. It can be applied to the skin, inhaled, or used in the nose. Mometasone furoate, not mometasone, is used in medical products.
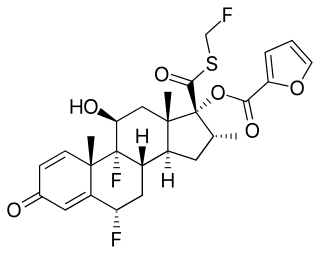
Fluticasone furoate, sold under the brand name Flonase Sensimist among others, is a corticosteroid for the treatment of non-allergic and allergic rhinitis administered by a nasal spray. It is also available as an inhaled corticosteroid to help prevent and control symptoms of asthma. It is derived from cortisol. Unlike fluticasone propionate, which is only approved for children four years and older, fluticasone furoate is approved in children as young as two years of age when used for allergies.
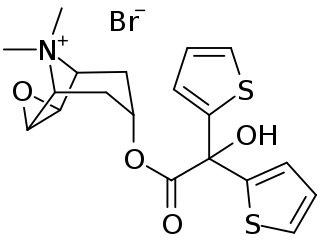
Tiotropium bromide, sold under the brand name Spiriva among others, is a long-acting bronchodilator used in the management of chronic obstructive pulmonary disease (COPD) and asthma. Specifically it is used during periods of breathing difficulty to prevent them from getting worse, rather than to prevent them from happening. It is used by inhalation through the mouth. Onset typically begins within half an hour and lasts for 24 hours.

Mometasone/formoterol, sold under the brand name Dulera among others, is a fixed-dose combination medication used in the long-term treatment of asthma. It contains mometasone a steroid and formoterol a long-acting beta agonist. It is only recommended in those for whom an inhaled steroid is not sufficient. It is used by inhalation.

Fluticasone furoate/vilanterol, sold under the brand name Breo Ellipta among others, is a combination medication for the treatment of chronic obstructive pulmonary disease (COPD) and asthma. It contains fluticasone furoate, an inhaled corticosteroid, and vilanterol, an ultra-long-acting β2 agonist (ultra-LABA).
Ellipta is part of GlaxoSmithKline's trade names of several inhalable asthma and chronic obstructive airway disease (COPD) combination medications that make use of the same type of inhaler:

Indacaterol/glycopyrronium bromide, sold under the brand name Ultibro Breezhaler among others, is a fixed-dose combination medication for inhalation consisting of the following two active ingredients:

Beclometasone/formoterol/glycopyrronium, sold under the brand name Trimbow among others, is an inhalable fixed-dose combination medication for the treatment of chronic obstructive pulmonary disease (COPD) and asthma. It contains beclometasone dipropionate, formoterol fumarate dihydrate, and glycopyrronium bromide.
Fluticasone furoate/umeclidinium bromide/vilanterol, sold under the brand name Trelegy Ellipta among others, is a fixed-dose combination inhaled medication that is used for the maintenance treatment of chronic obstructive pulmonary disease (COPD). The medications work in different ways: fluticasone furoate is an inhaled corticosteroid (ICS), umeclidinium is a long-acting muscarinic antagonist (LAMA), and vilanterol is a long-acting beta-agonist (LABA).
Glycopyrronium bromide/formoterol, sold under the brand name Bevespi Aerosphere, is a combination medication for the maintenance treatment of chronic obstructive pulmonary disease (COPD). It is a combination of glycopyrronium bromide and formoterol. It is inhaled.
Indacaterol/mometasone, sold under the brand name Atectura Breezhaler among others, is a fixed-dose combination medication for the treatment of asthma in adults and adolescents twelve years of age and older not adequately controlled with inhaled corticosteroids and inhaled short acting beta2 agonists.
References
- ↑ "Australian Public Assessment Report for Indacaterol (as acetate) / glycopyrronium (as bromide) / mometasone furoate" (PDF).
- ↑ "AusPAR: Indacaterol (as acetate) / glycopyrronium (as bromide) / mometasone furoate". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 29 April 2023.
- ↑ "TGA eBS - Product and Consumer Medicine Information Licence". Archived from the original on 13 June 2021. Retrieved 13 June 2021.
- ↑ "Enerzair Breezhaler (Novartis Pharmaceuticals Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 22 September 2022. Retrieved 29 April 2023.
- ↑ "Regulatory Decision Summary - Enerzair Breezhaler". Health Canada. 23 October 2014. Archived from the original on 7 June 2022. Retrieved 7 June 2022.
- ↑ "Enerzair Breezhaler - Summary of Product Characteristics (SmPC)". (emc). 12 May 2021. Archived from the original on 13 June 2021. Retrieved 12 June 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 "Enerzair Breezhaler EPAR". European Medicines Agency (EMA). 28 April 2020. Archived from the original on 22 July 2020. Retrieved 21 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 3 "Zimbus Breezhaler EPAR". European Medicines Agency (EMA). 28 April 2020. Archived from the original on 29 September 2020. Retrieved 2 September 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Enerzair Breezhaler Product information". Union Register of medicinal products. Archived from the original on 5 March 2023. Retrieved 3 March 2023.
- ↑ "Zimbus Breezhaler Product information". Union Register of medicinal products. Archived from the original on 5 March 2023. Retrieved 3 March 2023.
- 1 2 3 4 5 6 "First triple combination therapy for asthma with optional electronic sensor". European Medicines Agency (EMA). 30 April 2020. Archived from the original on 22 July 2020. Retrieved 21 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 "Novartis receives EC approval for Enerzair Breezhaler, including the first digital companion (sensor and app) that can be prescribed alongside a treatment for uncontrolled asthma in the EU". Novartis (Press release). 7 July 2020. Archived from the original on 22 July 2020. Retrieved 21 July 2020.
- ↑ "Novartis receives CHMP positive opinion for Enerzair Breezhaler (QVM149), a potential first-in-class inhaled LABA/LAMA/ICS combination for uncontrolled asthma". Novartis (Press release). 1 May 2020. Archived from the original on 22 July 2020. Retrieved 21 July 2020.
- ↑ "Zimbus Breezhaler: Pending EC decision". European Medicines Agency (EMA). 30 April 2020. Archived from the original on 22 July 2020. Retrieved 21 July 2020.