
Ephedrine is a central nervous system (CNS) stimulant and sympathomimetic agent that is often used to prevent low blood pressure during anesthesia. It has also been used for asthma, narcolepsy, and obesity but is not the preferred treatment. It is of unclear benefit in nasal congestion. It can be taken by mouth or by injection into a muscle, vein, or just under the skin. Onset with intravenous use is fast, while injection into a muscle can take 20 minutes, and by mouth can take an hour for effect. When given by injection, it lasts about an hour, and when taken by mouth, it can last up to four hours.

Methcathinone is a monoamine alkaloid and psychoactive stimulant, a substituted cathinone. It is used as a recreational drug due to its potent stimulant and euphoric effects and is considered to be addictive, with both physical and psychological withdrawal occurring if its use is discontinued after prolonged or high-dosage administration. It is usually snorted, but can be smoked, injected, or taken orally.

Phenylpropanolamine (PPA), sold under many brand names, is a sympathomimetic agent which is used as a decongestant and appetite suppressant. It was previously commonly used in prescription and over-the-counter cough and cold preparations. The medication is taken by mouth.

Fenfluramine, sold under the brand name Fintepla, is a serotonergic medication used for the treatment of seizures associated with Dravet syndrome and Lennox–Gastaut syndrome. It was formerly used as an appetite suppressant in the treatment of obesity, but was discontinued for this use due to cardiovascular toxicity before being repurposed for new indications. Fenfluramine was used for weight loss both alone under the brand name Pondimin and in combination with phentermine commonly known as fen-phen.

The dopamine transporter is a membrane-spanning protein coded for in humans by the SLC6A3 gene, that pumps the neurotransmitter dopamine out of the synaptic cleft back into cytosol. In the cytosol, other transporters sequester the dopamine into vesicles for storage and later release. Dopamine reuptake via DAT provides the primary mechanism through which dopamine is cleared from synapses, although there may be an exception in the prefrontal cortex, where evidence points to a possibly larger role of the norepinephrine transporter.

Levmetamfetamine, also known as l-desoxyephedrine or levomethamphetamine, and commonly sold under the brand name Vicks VapoInhaler among others, is an optical isomer of methamphetamine primarily used as a topical nasal decongestant. It is used to treat nasal congestion from allergies and the common cold. It was first used medically as decongestant beginning in 1958 and has been used for such purposes, primarily in the United States, since then.

Chlorphentermine is a serotonergic appetite suppressant of the amphetamine family. Developed in 1962, it is the 4-chloro derivative of the better known appetite suppressant phentermine, which is still in current use.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.
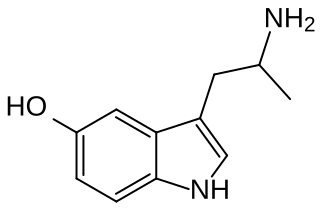
α-Methylserotonin (αMS), also known as α-methyl-5-hydroxytryptamine (α-methyl-5-HT) or 5-hydroxy-α-methyltryptamine (5-HO-αMT), is a tryptamine derivative closely related to the neurotransmitter serotonin (5-HT). It acts as a non-selective serotonin receptor agonist and has been used extensively in scientific research to study the function of the serotonin system.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons.
A dopamine releasing agent (DRA) is a type of drug which induces the release of dopamine in the body and/or brain. No selective and robust DRAs are currently known. On the other hand, many releasing agents of both dopamine and norepinephrine and of serotonin, norepinephrine, and dopamine are known. Serotonin–dopamine releasing agents (SDRAs), for instance 5-chloro-αMT, are much more rare and are not selective for dopamine release but have also been developed. Examples of major NDRAs include the psychostimulants amphetamine and methamphetamine, while an example of an SNDRA is the entactogen methylenedioxymethamphetamine (MDMA). These drugs are frequently used for recreational purposes and encountered as drugs of abuse. Selective DRAs, as well as NDRAs, have medical applications in the treatment of attention deficit hyperactivity disorder (ADHD).

Substituted cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

Levofenfluramine (INN), or (−)-3-trifluoromethyl-N-ethylamphetamine, also known as (−)-fenfluramine or (R)-fenfluramine, is a drug of the amphetamine family that, itself (i.e., in enantiopure form), was never marketed. It is the levorotatory enantiomer of fenfluramine, the racemic form of the compound, whereas the dextrorotatory enantiomer is dexfenfluramine. Both fenfluramine and dexfenfluramine are anorectic agents that have been used clinically in the treatment of obesity (and hence, levofenfluramine has been as well since it is a component of fenfluramine). However, they have since been discontinued due to reports of causing cardiovascular conditions such as valvular heart disease and pulmonary hypertension, adverse effects that are likely to be caused by excessive stimulation of 5-HT2B receptors expressed on heart valves.

Pseudophenmetrazine is a psychostimulant compound of the morpholine class. It is the N-demethylated and cis-configured analogue of phendimetrazine as well as the cis-configured stereoisomer of phenmetrazine. In addition, along with phenmetrazine, it is believed to be one of the active metabolites of phendimetrazine, which itself is inactive and behaves merely as a prodrug. Relative to phenmetrazine, pseudophenmetrazine is of fairly low potency, acting as a modest releasing agent of norepinephrine (EC50 = 514 nM), while its (+)-enantiomer is a weak releaser of dopamine (EC50 = 1,457 nM) whereas its (−)-enantiomer is a weak reuptake inhibitor of dopamine (Ki = 2,691 nM); together as a racemic mixture with the two enantiomers combined, pseudophenmetrazine behaves overall more as a dopamine reuptake inhibitor (Ki = 2,630 nM), possibly due to the (+)-enantiomer blocking the uptake of the (−)-enantiomer into dopaminergic neurons and thus preventing it from inducing dopamine release. Neither enantiomer has any significant effect on serotonin reuptake or release (both Ki = >10,000 nM and EC50 = >10,000 nM, respectively).

L-Norpseudoephedrine, or (−)-norpseudoephedrine, is a psychostimulant drug of the amphetamine family. It is one of the four optical isomers of phenylpropanolamine, the other three being cathine ((+)-norpseudoephedrine), (−)-norephedrine, and (+)-norephedrine; as well as one of the two enantiomers of norpseudoephedrine (the other being cathine). Similarly to cathine, L-norpseudoephedrine acts as a releasing agent of norepinephrine (EC50 = 30 nM) and to a lesser extent of dopamine (EC50 = 294 nM). Due to the 10-fold difference in its potency for inducing the release of the two neurotransmitters however, L-norpseudoephedrine could be called a modestly selective or preferential norepinephrine releasing agent, similarly to related compounds like ephedrine and pseudoephedrine.

Methamnetamine is a triple monoamine releasing agent and N-methyl analog of the non-neurotoxic experimental drug naphthylaminopropane and the naphthalene analog of methamphetamine. It has been sold online as a designer drug.

ortho-Methylphenylpiperazine (also known as oMPP, oMePP, 1-(2-methylphenyl)piperazine, 2-MPP, and 2-MePP) is a psychoactive designer drug of the phenylpiperazine group. It acts as a serotonin–norepinephrine–dopamine releasing agent (SNDRA), with EC50 values for induction of monoamine release of 175 nM for serotonin, 39.1 nM for norepinephrine, and 296–542 nM for dopamine. As such, it has about 4.5-fold preference for induction of norepinephrine release over serotonin, and about 7.6- to 13.9-fold preference for induction of norepinephrine release over dopamine.

Substituted β-hydroxyamphetamines, also known as substituted phenylisopropanolamines, substituted phenylpropanolamines, substituted norephedrines, or substituted cathinols, are derivatives of β-hydroxyamphetamine with one or more chemical substituents. They are substituted phenethylamines, phenylethanolamines (β-hydroxyphenethylamines), and amphetamines (α-methylphenethylamines), and are closely related to but distinct from the substituted cathinones (β-ketoamphetamines). Examples of β-hydroxyamphetamines include the β-hydroxyamphetamine stereoisomers phenylpropanolamine and cathine and the stereospecific N-methylated β-hydroxyamphetamine derivatives ephedrine and pseudoephedrine, among many others.

















