 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Daxas, Daliresp, Zoryve, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611034 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, topical |
| Drug class | PDE4 inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 79% [4] [3] [8] [9] |
| Protein binding | 99% [4] [3] [8] [9] |
| Metabolism | Hepatic via CYP1A2 & CYP3A4 [4] [3] [8] [9] |
| Elimination half-life | 17 hours (30 hours [active metabolite]) [4] [3] [8] [9] |
| Excretion | Urine (70%) [4] [3] [8] [9] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.960 |
| Chemical and physical data | |
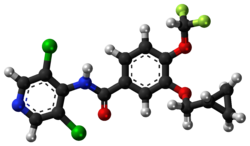
| Formula | C17H14Cl2F2N2O3 |
| Molar mass | 403.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Roflumilast, sold under the brand name Daxas among others, is a medication used for the treatment of chronic obstructive pulmonary disease, [4] plaque psoriasis, [5] seborrheic dermatitis, [6] and atopic dermatitis. [5] It acts as a selective, long-acting inhibitor of the enzyme phosphodiesterase-4 (PDE-4). [10] It has anti-inflammatory effects. [10] [11] [12]
Contents
It was approved for medical use in the European Union in 2010, [7] in the United States in 2011, [4] and in Canada in 2017. [1] It is available as a generic medication. [13]