
CC-1088 is a thalidomide analogue inhibitor of phosphodiesterase 4 that was being developed up to 2005 by Celgene Corp., for treating of inflammatory diseases and myelodysplastic syndromes. [1] Apremilast (CC-10004) was found to be a preferable. [2]

CC-1088 is a thalidomide analogue inhibitor of phosphodiesterase 4 that was being developed up to 2005 by Celgene Corp., for treating of inflammatory diseases and myelodysplastic syndromes. [1] Apremilast (CC-10004) was found to be a preferable. [2]

Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality".

Thalidomide, sold under the brand names Contergan and Thalomid among others, is a medication used to treat a number of cancers, graft-versus-host disease, and a number of skin conditions including complications of leprosy. While it has been used in a number of HIV associated conditions, such use is associated with increased levels of the virus. It is administered orally.
Teratology is the study of abnormalities of physiological development. It is often thought of as the study of human congenital abnormalities, but it is broader than that, taking into account other non-birth developmental stages, including puberty; and other organisms, including plants. The related term developmental toxicity includes all manifestations of abnormal development that are caused by environmental insult. These may include growth retardation, delayed mental development or other congenital disorders without any structural malformations.
In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic form. Half of the optically active substance becomes its mirror image (enantiomer) referred as racemic mixtures. If the racemization results in a mixture where the D and L enantiomers are present in equal quantities, the resulting sample is described as a racemic mixture or a racemate. Racemization can proceed through a number of different mechanisms, and it has particular significance in pharmacology as different enantiomers may have different pharmaceutical effects.

Grünenthal is a pharmaceutical company headquartered in Aachen in Germany. The company was founded in 1946 as Chemie Grünenthal and has been continuously family-owned. The company was the first to introduce penicillin into the German market in the postwar period, after the Allied Control Council lifted its ban.
William Griffith McBride CBE AO was an Australian obstetrician. He discovered the teratogenicity of thalidomide, which resulted in the reduction of the number of drugs prescribed during pregnancy. McBride was found guilty of separate counts of medical malpractice and scientific fraud

Frances Kathleen Oldham Kelsey, CM was a Canadian-American pharmacologist and physician. As a reviewer for the U.S. Food and Drug Administration (FDA), she refused to authorize thalidomide for market because she had concerns about the lack of evidence regarding the drug's safety. Her concerns proved to be justified when it was shown that thalidomide caused serious birth defects. Kelsey's career intersected with the passage of laws strengthening FDA oversight of pharmaceuticals. Awarded by John F. Kennedy, Kelsey was the second woman to receive the President's Award for Distinguished Federal Civilian Service.

Lenalidomide, sold under the trade name Revlimid among others, is a medication used to treat multiple myeloma (MM) and myelodysplastic syndromes (MDS). For MM it is used after at least one other treatment and generally together with dexamethasone. It is taken by mouth.
Moses Judah Folkman was an American medical scientist best known for his research on tumor angiogenesis, the process by which a tumor attracts blood vessels to nourish itself and sustain its existence. He founded the field of angiogenesis research, which has led to the discovery of a number of therapies based on inhibiting or stimulating neovascularization.

Celgene Corporation is a pharmaceutical company that makes cancer and immunology drugs. Its major product is Revlimid (lenalidomide), which is used in the treatment of multiple myeloma, and also in certain anemias. The company is incorporated in Delaware, headquartered in Summit, New Jersey, and a subsidiary of Bristol Myers Squibb (BMS).
Protein-bound paclitaxel, also known as nanoparticle albumin–bound paclitaxel or nab-paclitaxel, is an injectable formulation of paclitaxel used to treat breast cancer, lung cancer and pancreatic cancer, among others. Paclitaxel kills cancer cells by preventing the normal breakdown of microtubules during cell division. In this formulation, paclitaxel is bonded to albumin as a delivery vehicle. It is manufactured and sold in the United States by Celgene under the trade name Abraxane where it is designated as an orphan drug as first-line treatment, in combination with gemcitabine, for the orphan disease "metastatic adenocarcinoma of the pancreas".

Pomalidomide is a derivative of thalidomide marketed by Celgene. It is anti-angiogenic and also acts as an immunomodulator.

Professor Jacob Sheskin, sometimes known as Sheskin Jacob was an Israeli physician best known for his 1964 serendipitous discovery that thalidomide can be used as a treatment for leprosy at Hadassah University in Jerusalem.

In the late 1950s and early 1960s, the use of thalidomide in pregnant women in 46 countries resulted in the "biggest man‐made medical disaster ever", resulting in more than 10,000 children born with a range of severe deformities, such as phocomelia, as well as thousands of miscarriages.

A phosphodiesterase type 4 inhibitor, commonly referred to as a PDE4 inhibitor, is a drug used to block the degradative action of phosphodiesterase 4 (PDE4) on cyclic adenosine monophosphate (cAMP). It is a member of the larger family of PDE inhibitors. The PDE4 family of enzymes are the most prevalent PDE in immune cells. They are predominantly responsible for hydrolyzing cAMP within both immune cells and cells in the central nervous system.
Fedratinib (trade name Inrebic), used in the form of fedratinib hydrochloride, is an orally available semi-selective inhibitor of Janus kinase 2 (JAK-2) developed for the treatment of patients with myeloproliferative diseases including myelofibrosis. Fedratinib acts as a competitive inhibitor of protein kinase JAK-2 with IC50=6 nM; related kinases FLT3 and RET are also sensitive, with IC50=25 nM and IC50=17 nM, respectively. Significantly less activity was observed against other tyrosine kinases including JAK3 (IC50=169 nM). In treated cells the inhibitor blocks downstream cellular signalling (JAK-STAT) leading to suppression of proliferation and induction of apoptosis. It was approved by FDA on 16 August 2019.
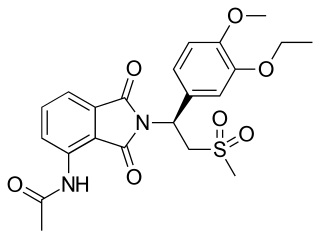
Apremilast, sold under the brand name Otezla among others, is a medication for the treatment of certain types of psoriasis and psoriatic arthritis. It may also be useful for other immune system related inflammatory diseases. The drug acts as a selective inhibitor of the enzyme phosphodiesterase 4 (PDE4) and inhibits spontaneous production of TNF-alpha from human rheumatoid synovial cells. It is taken by mouth.

Immunomodulatory imide drugs (IMiDs) are a class of immunomodulatory drugs containing an imide group. The IMiD class includes thalidomide and its analogues. These drugs may also be referred to as 'Cereblon modulators'. Cereblon is the protein targeted by this class of drugs.

Ozanimod, sold under the brand name Zeposia, is an immunomodulatory drug for the treatment of relapsing multiple sclerosis (RMS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. It acts as a sphingosine-1-phosphate (S1P) receptor agonist, sequestering lymphocytes to peripheral lymphoid organs and away from their sites of chronic inflammation. Ozanimod was discovered by The Scripps Research Institute in San Diego, by the labs of Hugh Rosen and Edward Roberts, and licensed to the biotech company Receptos Inc. Receptos was acquired by Celgene for $7.2 billion.
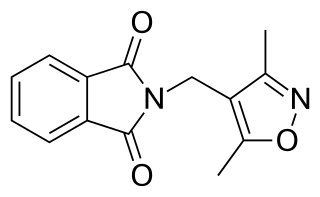
DIMP, or N-(3,5-dimethyl-4-isoxazolylmethyl)phthalimide, is a nonsteroidal antiandrogen (NSAA) structurally related to thalidomide that was first described in 1973 and was never marketed. Along with flutamide, it was one of the earliest NSAAs to be discovered, and for this reason, has been described as a "classical" NSAA. The drug is a selective, competitive, silent antagonist of the AR, although it is described as an "only relatively weak competitor". Its relative binding affinity for the androgen receptor is about 2.6% of that of metribolone. DIMP possesses no androgenic, estrogenic, progestogenic, or antigonadotropic activity, but it does reverse the antigonadotropic effects of testosterone, indicating that, like other pure AR antagonists, it is progonadotropic.