
A phosphodiesterase inhibitor is a drug that blocks one or more of the five subtypes of the enzyme phosphodiesterase (PDE), thereby preventing the inactivation of the intracellular second messengers, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) by the respective PDE subtype(s). The ubiquitous presence of this enzyme means that non-specific inhibitors have a wide range of actions, the actions in the heart, and lungs being some of the first to find a therapeutic use.
A phosphodiesterase (PDE) is an enzyme that breaks a phosphodiester bond. Usually, phosphodiesterase refers to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many other families of phosphodiesterases, including phospholipases C and D, autotaxin, sphingomyelin phosphodiesterase, DNases, RNases, and restriction endonucleases, as well as numerous less-well-characterized small-molecule phosphodiesterases.
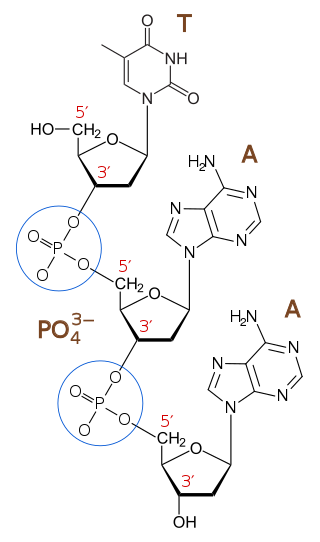
In chemistry, a phosphodiester bond occurs when exactly two of the hydroxyl groups in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. The "bond" involves this linkage C−O−PO−2O−C. Discussion of phosphodiesters is dominated by their prevalence in DNA and RNA, but phosphodiesters occur in other biomolecules, e.g. acyl carrier proteins.

A phosphodiesterase type 5 inhibitor is a vasodilating drug that works by blocking the degradative action of cGMP-specific phosphodiesterase type 5 (PDE5) on cyclic GMP in the smooth muscle cells lining the blood vessels supplying various tissues. These drugs dilate the corpora cavernosa of the penis, facilitating erection with sexual stimulation, and are used in the treatment of erectile dysfunction (ED). Sildenafil was the first effective oral treatment available for ED. Because PDE5 is also present in the smooth muscle of the walls of the arterioles within the lungs, two PDE5 inhibitors, sildenafil and tadalafil, are FDA-approved for the treatment of pulmonary hypertension. As of 2019, the wider cardiovascular benefits of PDE5 inhibitors are being appreciated.

Cyclic guanosine monophosphate-specific phosphodiesterase type 5 is an enzyme from the phosphodiesterase class. It is found in various tissues, most prominently the corpus cavernosum and the retina. It has also been recently discovered to play a vital role in the cardiovascular system.

Aminophylline is a compound of the bronchodilator theophylline with ethylenediamine in 2:1 ratio. The ethylenediamine improves solubility, and the aminophylline is usually found as a dihydrate.

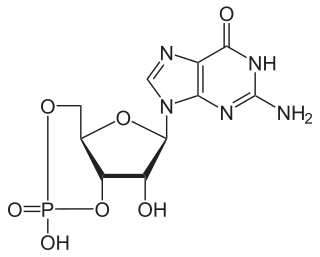
3′,5′-cyclic-nucleotide phosphodiesterases (EC 3.1.4.17) are a family of phosphodiesterases. Generally, these enzymes hydrolyze a nucleoside 3′,5′-cyclic phosphate to a nucleoside 5′-phosphate:

Rolipram is a selective phosphodiesterase-4 inhibitor discovered and developed by Schering AG as a potential antidepressant drug in the early 1990s. It served as a prototype molecule for several companies' drug discovery and development efforts. Rolipram was discontinued after clinical trials showed that its therapeutic window was too narrow; it could not be dosed at high enough levels to be effective without causing significant gastrointestinal side effects.
EHNA is a potent adenosine deaminase inhibitor, which also acts as a phosphodiesterase inhibitor that selectively inhibits phosphodiesterase type 2 (PDE2).

PDE3 is a phosphodiesterase. The PDEs belong to at least eleven related gene families, which are different in their primary structure, substrate affinity, responses to effectors, and regulation mechanism. Most of the PDE families are composed of more than one gene. PDE3 is clinically significant because of its role in regulating heart muscle, vascular smooth muscle and platelet aggregation. PDE3 inhibitors have been developed as pharmaceuticals, but their use is limited by arrhythmic effects and they can increase mortality in some applications.
Phosphodiesterase 1, PDE1, EC 3.1.4.1, systematic name oligonucleotide 5′-nucleotidohydrolase) is a phosphodiesterase enzyme also known as calcium- and calmodulin-dependent phosphodiesterase. It is one of the 11 families of phosphodiesterase (PDE1-PDE11). Phosphodiesterase 1 has three subtypes, PDE1A, PDE1B and PDE1C which divide further into various isoforms. The various isoforms exhibit different affinities for cAMP and cGMP.

The PDE2 enzyme is one of 21 different phosphodiesterases (PDE) found in mammals. These different PDEs can be subdivided to 11 families. The different PDEs of the same family are functionally related despite the fact that their amino acid sequences show considerable divergence. The PDEs have different substrate specificities. Some are cAMP selective hydrolases, others are cGMP selective hydrolases and the rest can hydrolyse both cAMP and cGMP.
Autotaxin, also known as ectonucleotide pyrophosphatase/phosphodiesterase family member 2, is an enzyme that in humans is encoded by the ENPP2 gene.
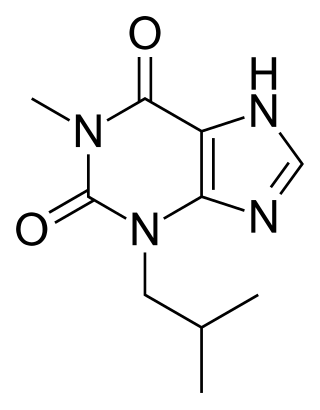
IBMX (3-isobutyl-1-methylxanthine), like other methylated xanthine derivatives, is both a:
- competitive non-selective phosphodiesterase inhibitor which raises intracellular cAMP, activates PKA, inhibits TNFα and leukotriene synthesis, and reduces inflammation and innate immunity, and
- nonselective adenosine receptor antagonist.

Ibudilast is an anti-inflammatory drug used mainly in Japan, which acts as a phosphodiesterase inhibitor, inhibiting the PDE4 subtype to the greatest extent, but also showing significant inhibition of other PDE subtypes.

cAMP-specific 3',5'-cyclic phosphodiesterase 4A is an enzyme that in humans is encoded by the PDE4A gene.

cAMP-specific 3',5'-cyclic phosphodiesterase 4B is an enzyme that in humans is encoded by the PDE4B gene.

Etazolate (SQ-20,009, EHT-0202) is an anxiolytic drug which is a pyrazolopyridine derivative and has unique pharmacological properties. It acts as a positive allosteric modulator of the GABAA receptor at the barbiturate binding site, as an adenosine antagonist of the A1 and A2 subtypes, and as a phosphodiesterase inhibitor selective for the PDE4 isoform. It is currently in clinical trials for the treatment of Alzheimer's disease.

A phosphodiesterase-4 inhibitor, commonly referred to as a PDE4 inhibitor, is a drug used to block the degradative action of phosphodiesterase 4 (PDE4) on cyclic adenosine monophosphate (cAMP). It is a member of the larger family of PDE inhibitors. The PDE4 family of enzymes are the most prevalent PDE in immune cells. They are predominantly responsible for hydrolyzing cAMP within both immune cells and cells in the central nervous system.
3′,5′-cyclic-AMP phosphodiesterase (EC 3.1.4.53, cAMP-specific phosphodiesterase, cAMP-specific PDE, PDE1, PDE2A, PDE2B, PDE4, PDE7, PDE8, PDEB1, PDEB2) is an enzyme with systematic name 3′,5′-cyclic-AMP 5′-nucleotidohydrolase. It catalyses the following reaction
















