| |
| Clinical data | |
|---|---|
| Trade names | Bronica in Japan, Changnuo, Mai Xu Jia, Quan Kang Nuo in China and as Seradair in India. . [1] |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth (tablets, granules) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | >96% |
| Elimination half-life | 22 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.220.176 |
| Chemical and physical data | |
| Formula | C22H26O4 |
| Molar mass | 354.446 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Seratrodast (development name, AA-2414; marketed originally as Bronica) [2] is a thromboxane A2 (TXA2) receptor (TP receptor) antagonist used primarily in the treatment of asthma. [3] [4] It was the first TP receptor antagonist that was developed as an anti-asthmatic drug and received marketing approval in Japan in 1997. [5] As of 2017 seratrodast was marketed as Bronica in Japan, and as Changnuo, Mai Xu Jia, Quan Kang Nuo in China. [1]
Contents
- Medical uses
- Contraindications and interactions
- Adverse effects
- Pharmacology
- Pharmacokinetics
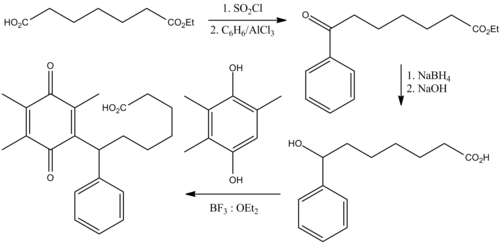
- Chemistry
- History
- Society and culture
- Research
- References
Unlike thromboxane synthase inhibitors such as ozagrel, seratrodast does not affect thrombus formation, time to occlusion and bleeding time. [6] Seratrodast has no effect on prothrombin time and activated partial thromboplastin time, thus ruling out any action on blood coagulation cascade. [7]