| |||
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| KEGG | |||
| MeSH | Thromboxane+A2 | ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C20H32O5 | |||
| Molar mass | 352.471 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
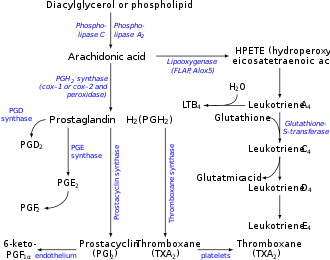
Thromboxane A2 (TXA2) is a type of thromboxane that is produced by activated platelets during hemostasis and has prothrombotic properties: it stimulates activation of new platelets as well as increases platelet aggregation. This is achieved by activating the thromboxane receptor, which results in platelet-shape change, inside-out activation of integrins, and degranulation. [1] Circulating fibrinogen binds these receptors on adjacent platelets, further strengthening the clot. TXA2 is also a known vasoconstrictor [2] [3] [4] [5] and is especially important during tissue injury and inflammation. It is also regarded as responsible for Prinzmetal's angina.[ citation needed ]
Receptors that mediate TXA2 actions are thromboxane A2 receptors. The human TXA2 receptor (TP) is a typical G protein-coupled receptor (GPCR) with seven transmembrane segments. In humans, two TP receptor splice variants – TPα and TPβ – have so far been cloned.