Silicon tetrachloride or tetrachlorosilane is the inorganic compound with the formula SiCl4. It is a colorless volatile liquid that fumes in air. It is used to produce high purity silicon and silica for commercial applications. It is a part of the chlorosilane family.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as “tickle” or “tickle 4”, as a phonetic representation of the symbols of its molecular formula.

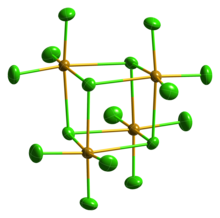
Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride, is an inorganic compound with the formula SnCl4. It is a colorless hygroscopic liquid, which fumes on contact with air. It is used as a precursor to other tin compounds. It was first discovered by Andreas Libavius (1550–1616) and was known as spiritus fumans libavii.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It has two forms, the monohydrate (AuCl3·H2O) and the anhydrous form, which are both hygroscopic and light-sensitive solids. This compound is a dimer of AuCl3. This compound has a few uses, such as an oxidizing agent and for catalyzing various organic reactions.
In inorganic chemistry, chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond. Trichlorosilane is produced on the largest scale. The parent chlorosilane is silicon tetrachloride.

Scandium(III) chloride is the inorganic compound with the formula ScCl3. It is a white, high-melting ionic compound, which is deliquescent and highly water-soluble. This salt is mainly of interest in the research laboratory. Both the anhydrous form and hexahydrate (ScCl3•6H2O) are commercially available.

Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate.

Tellurium tetrachloride is the inorganic compound with the empirical formula TeCl4. The compound is volatile, subliming at 200 °C at 0.1 mmHg. Molten TeCl4 is ionic, dissociating into TeCl3+ and Te2Cl102−.

Platinum(IV) chloride is the inorganic compound of platinum and chlorine with the empirical formula PtCl4. This brown solid features platinum in the 4+ oxidation state.
Technetium compounds are chemical compounds containing the chemical element technetium. Technetium can form multiple oxidation states, but often forms in the +4 and +7 oxidation states. Because technetium is radioactive, technetium compounds are extremely rare on Earth.

Germanium dichloride is a chemical compound of germanium and chlorine with the formula GeCl2. It is a yellow solid. Germanium dichloride is an example of a compound featuring germanium in the +2 oxidation state.
Selenium monochloride or diselenium dichloride is an inorganic compound with the formula Se2Cl2. Although a common name for the compound is selenium monochloride, reflecting its empirical formula, IUPAC does not recommend that name, instead preferring the more descriptive diselenium dichloride.

Sulfur tetrachloride is an inorganic compound with chemical formula SCl4. It has only been obtained as an unstable pale yellow solid. The corresponding SF4 is a stable, useful reagent.

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.

Lead tetrachloride, also known as lead(IV) chloride, has the molecular formula PbCl4. It is a yellow, oily liquid which is stable below 0 °C, and decomposes at 50 °C. It has a tetrahedral configuration, with lead as the central atom. The Pb–Cl covalent bonds have been measured to be 247 pm and the bond energy is 243 kJ⋅mol−1.
Indium(II) chloride is an inorganic compound, an indium metal salt and hydrochloric acid with the formula InCl2. The compound forms colorless crystals, reacts with water. This is one of three known indium chlorides.