
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as "tickle" or "tickle 4" due to the phonetic resemblance of its molecular formula to the word.

Scandium(III) chloride is the inorganic compound with the formula ScCl3. It is a white, high-melting ionic compound, which is deliquescent and highly water-soluble. This salt is mainly of interest in the research laboratory. Both the anhydrous form and hexahydrate (ScCl3•6H2O) are commercially available.

Chromium(II) chloride describes inorganic compounds with the formula CrCl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue air-sensitive solutions of the tetrahydrate Cr(H2O)4Cl2. Chromium(II) chloride has no commercial uses but is used on a laboratory-scale for the synthesis of other chromium complexes.

Vanadium oxytrichloride is the inorganic compound with the formula VOCl3. This yellow distillable liquid hydrolyzes readily in air. It is an oxidizing agent. It is used as a reagent in organic synthesis. Samples often appear red or orange owing to an impurity of vanadium tetrachloride.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.

Vanadium(III) chloride is the inorganic compound with the formula VCl3 which forms the hexahydrate, [VCl2(H2O)4]Cl·2H2O. This hygroscopic purple salt is a common precursor to other vanadium(III) complexes.

Tungsten hexachloride is an inorganic chemical compound of tungsten and chlorine with the chemical formula WCl6. This dark violet blue compound exists as volatile crystals under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known.

Titanium tetraiodide is an inorganic compound with the formula TiI4. It is a black volatile solid, first reported by Rudolph Weber in 1863. It is an intermediate in the van Arkel–de Boer process for the purification of titanium.
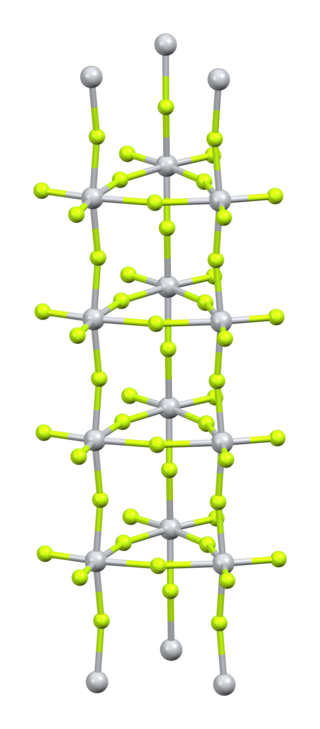
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure. In common with the other tetrahalides, TiF4 is a strong Lewis acid.
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.
Gregory S. Girolami is the William H. and Janet G. Lycan Professor of Chemistry at the University of Illinois at Urbana-Champaign. His research focuses on the synthesis, properties, and reactivity of new inorganic, organometallic, and solid state species. Girolami has been elected a fellow of the American Association for the Advancement of Science, the Royal Society of Chemistry, and the American Chemical Society.

Transition metal alkyl complexes are coordination complexes that contain a bond between a transition metal and an alkyl ligand. Such complexes are not only pervasive but are of practical and theoretical interest.
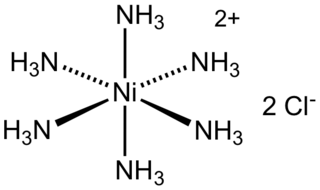
Hexaamminenickel chloride is the chemical compound with the formula [Ni(NH3)6]Cl2. It is the chloride salt of the metal ammine complex [Ni(NH3)6]2+. The cation features six ammonia (called ammines in coordination chemistry) ligands attached to the nickel(II) ion.

Vanadium oxydichloride is the inorganic compound with the formula VOCl2. One of several oxychlorides of vanadium, it is a hygroscopic green solid. It is prepared by comproportionation of vanadium trichloride and vanadium(V) oxides:

Europium(II) chloride is an inorganic compound with a chemical formula EuCl2. When it is irradiated by ultraviolet light, it has bright blue fluorescence.

Transition metal pyridine complexes encompass many coordination complexes that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridines, quinolines, and more complex rings.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.

Vanadium (V) chloride chlorimide is a chemical compound containing vanadium in a +5 oxidation state bound to three chlorine atoms and with a double bond to a chlorimide group (=NCl). It has formula VNCl4. This can be also considered as a chloroiminato complex.
A chloride nitride is a mixed anion compound containing both chloride (Cl−) and nitride ions (N3−). Another name is metallochloronitrides. They are a subclass of halide nitrides or pnictide halides.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.


















