| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC names Antimony pentachloride Antimony(V) chloride | |||
| Other names Antimonic chloride Antimony perchloride | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.729 | ||
| EC Number |
| ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| Cl5Sb | |||
| Molar mass | 299.01 g·mol−1 | ||
| Appearance | colorless or reddish-yellow (fuming) liquid, oily | ||
| Odor | pungent, offensive | ||
| Density | 2.336 g/cm3 (20 °C) [1] 2.36 g/cm3 (25 °C) [2] | ||
| Melting point | 2.8 °C (37.0 °F; 275.9 K) | ||
| Boiling point | 140 °C (284 °F; 413 K) decomposes from 106 °C [3] 79 °C (174 °F; 352 K) at 22 mmHg [1] 92 °C (198 °F; 365 K) at 30 mmHg [2] | ||
| reacts | |||
| Solubility | soluble in alcohol, HCl, tartaric acid, CHCl3, CS2, CCl4 | ||
| Solubility in selenium(IV) oxychloride | 62.97 g/100 g (25 °C) | ||
| Vapor pressure | 0.16 kPa (25 °C) 4 kPa (40 °C) 7.7 kPa (100 °C) [4] | ||
| −120.0·10−6 cm3/mol | |||
Refractive index (nD) | 1.59255 | ||
| Viscosity | 2.034 cP (29.4 °C) [1] 1.91 cP (35 °C) | ||
| Structure | |||
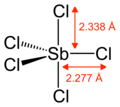
| Trigonal bipyramidal | |||
| 0 D | |||
| Thermochemistry [3] | |||
Heat capacity (C) | 120.9 J/mol·K (gas) | ||
Std molar entropy (S⦵298) | 295 J/mol·K | ||
Std enthalpy of formation (ΔfH⦵298) | −437.2 kJ/mol | ||
Gibbs free energy (ΔfG⦵) | −345.35 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Inhalation hazards | Toxic | ||
| GHS labelling: [2] | |||
    | |||
| Danger | |||
| H314, H411 | |||
| P273, P280, P305+P351+P338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 77 °C (171 °F; 350 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 1115 mg/kg, (rat, oral) [3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | TWA 0.5 mg/m3 (as Sb) [5] | ||
REL (Recommended) | TWA 0.5 mg/m3 (as Sb) [5] | ||
| Related compounds | |||
Other anions | Antimony pentafluoride | ||
Other cations | Phosphorus pentachloride | ||
Related compounds | Antimony trichloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers.