
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium.

n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry.

Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This vivid orange, opaque, crystalline explosive is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.

Methyllithium is the simplest organolithium reagent, with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive towards water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.

Tris(pentafluorophenyl)borane, sometimes referred to as "BCF", is the chemical compound (C6F5)3B. It is a white, volatile solid. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the BC3 core is planar. It has been described as the “ideal Lewis acid” because of its high thermal stability and the relative inertness of the B-C bonds. Related fluoro-substituted boron compounds, such as those containing B−CF3 groups, decompose with formation of B-F bonds. Tris(pentafluorophenyl)borane is thermally stable at temperatures well over 200 °C, resistant to oxygen, and water-tolerant.

Decamethyldizincocene is an organozinc compound with the formula [Zn2(η5–C5Me5)2]. It is the first and an unusual example of a compound with a Zn-Zn bond. Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the presence of oxygen and reacts with water. It is stable at room temperature and especially soluble in diethyl ether, benzene, pentane, or tetrahydrofuran.
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsane and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known.

Tetramethyltin is an organometallic compound with the formula (CH3)4Sn. This liquid, one of the simplest organotin compounds, is useful for transition-metal mediated conversion of acid chlorides to methyl ketones and aryl halides to aryl methyl ketones. It is volatile and toxic, so care should be taken when using it in the laboratory.
Organoantimony chemistry is the chemistry of compounds containing a carbon to antimony (Sb) chemical bond. Relevant oxidation states are SbV and SbIII. The toxicity of antimony limits practical application in organic chemistry.
Organorhenium chemistry describes the compounds with Re−C bonds. Because rhenium is a rare element, relatively few applications exist, but the area has been a rich source of concepts and a few useful catalysts.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.

Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.

1,2-Dimethyldiborane is an organoboron compound with the formula [(CH3)BH2]2. Structurally, it is related to diborane, but with methyl groups replacing terminal hydrides on each boron. It is the dimer of methylborane, CH3BH2, the simplest alkylborane. 1,2-Dimethyldiborane can exist in a cis- and a trans arrangement. 1,2-Dimethyldiborane is an easily condensed, colorless gas that ignites spontaneously in air.
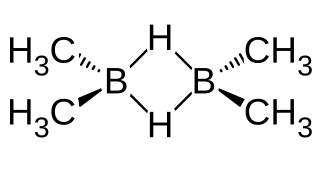
Dimethylborane, (CH3)2BH is the simplest dialkylborane, consisting of a methyl group substituted for a hydrogen in borane. As for other boranes it normally exists in the form of a dimer called tetramethyldiborane or tetramethylbisborane or TMDB ((CH3)2BH)2. Other combinations of methylation occur on diborane, including monomethyldiborane, trimethyldiborane, 1,2-dimethylborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.

Trimethyldiborane, (CH3)3B2H3 is a molecule containing boron carbon and hydrogen. It is an alkylborane, consisting of three methyl group substituted for a hydrogen in diborane. It can be considered a mixed dimer: (CH3)2BH2BH(CH3) or dimethylborane and methylborane. called 1,2-dimethyldiborane. Other combinations of methylation occur on diborane, including monomethyldiborane, 1,2-dimethyldiborane, tetramethyldiborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms, so it is difficult to keep it pure. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.
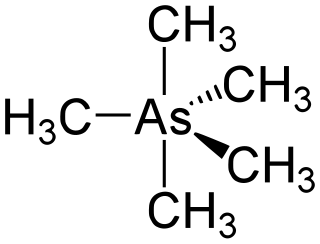
Pentamethylarsenic (or pentamethylarsorane) is an organometalllic compound containing five methyl groups bound to an arsenic atom with formula As(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid.

Pentamethylbismuth (or pentamethylbismuthorane) is an organometalllic compound containing five methyl groups bound to a bismuth atom with formula Bi(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid.

Pentamethyltantalum is a homoleptic organotantalum compound. It has a propensity to explode when it is melted. Its discovery was part of a sequence that led to Richard R. Schrock's Nobel Prize winning discovery in olefin metathesis.

Pentaphenylantimony is an organoantimony compound containing five phenyl groups attached to one antimony atom. It has formula Sb(C6H5)5 (or SbPh5).
Germyl, trihydridogermanate(1-), trihydrogermanide, trihydridogermyl or according to IUPAC Red Book: germanide is an anion containing germanium bounded with three hydrogens, with formula GeH−3. Germyl is the IUPAC term for the –GeH3 group. For less electropositive elements the bond can be considered covalent rather than ionic as "germanide" indicates. Germanide is the base for germane when it loses a proton.
















