In chemistry, an oxonium ion is any cation containing an oxygen atom that has three bonds and +1 formal charge. The simplest oxonium ion is the hydronium ion H3O+.
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group -COCl. Their formula is usually written RCOCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene. It is an alkylating agent, which makes it both useful and hazardous to handle.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as "tickle" or "tickle 4" due to the phonetic resemblance of its molecular formula to the word.
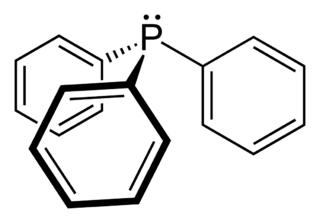
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Scandium(III) chloride is the inorganic compound with the formula ScCl3. It is a white, high-melting ionic compound, which is deliquescent and highly water-soluble. This salt is mainly of interest in the research laboratory. Both the anhydrous form and hexahydrate (ScCl3•6H2O) are commercially available.

Zirconium(IV) chloride, also known as zirconium tetrachloride, is an inorganic compound frequently used as a precursor to other compounds of zirconium. This white high-melting solid hydrolyzes rapidly in humid air.
Uranium borohydride is the inorganic compound with the empirical formula U(BH4)4. Two polymeric forms are known, as well as a monomeric derivative that exists in the gas phase. Because the polymers convert to the gaseous form at mild temperatures, uranium borohydride once attracted much attention. It is solid green.

Organoactinide chemistry is the science exploring the properties, structure and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.

Hexamethyltungsten is the chemical compound W(CH3)6 also written WMe6. Classified as a transition metal alkyl complex, hexamethyltungsten is an air-sensitive, red, crystalline solid at room temperature; however, it is extremely volatile and sublimes at −30 °C. Owing to its six methyl groups it is extremely soluble in petroleum, aromatic hydrocarbons, ethers, carbon disulfide, and carbon tetrachloride.

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organmetallic group 2 compounds are rare and are typically limited to academic interests.
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsane and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known.

Organobismuth chemistry is the chemistry of organometallic compounds containing a carbon to bismuth chemical bond. Applications are few. The main bismuth oxidation states are Bi(III) and Bi(V) as in all higher group 15 elements. The energy of a bond to carbon in this group decreases in the order P > As > Sb > Bi. The first reported use of bismuth in organic chemistry was in oxidation of alcohols by Challenger in 1934 (using Ph3Bi(OH)2). Knowledge about methylated species of bismuth in environmental and biological media is limited.

Tellurium dichloride is a chloride of tellurium with the chemical formula TeCl2.

Pentamethylantimony or pentamethylstiborane is an organometalllic compound containing five methyl groups bound to an antimony atom with formula Sb(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid. Some other antimony(V) organometallic compounds include pentapropynylantimony (Sb(CCCH3)5) and pentaphenyl antimony (Sb(C6H5)5). Other known pentamethyl-pnictides include pentamethylbismuth and pentamethylarsenic.

Pentamethylbismuth (or pentamethylbismuthorane) is an organometalllic compound containing five methyl groups bound to a bismuth atom with formula Bi(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid.

Pentamethyltantalum is a homoleptic organotantalum compound. It has a propensity to explode when it is melted. Its discovery was part of a sequence that lead to Richard R. Schrock's Nobel Prize discovery in olefin metathesis.
Triethylphosphine is the organophosphorus compound with the formula P(CH2CH3)3, commonly abbreviated as PEt3. It is a colorless liquid with an unpleasant odor characteristic of alkylphosphines. The compound is a common ligand in organometallic chemistry, such as in auranofin.
Magnesocene, also known as bis(cyclopentadienyl)magnesium(II) and sometimes abbreviated as MgCp2, is an organometallic compound with the formula Mg(η5-C5H5)2. It is an example of an s-block main group sandwich compound, structurally related to the d-block element metallocenes, and consists of a central magnesium atom sandwiched between two cyclopentadienyl rings.

An N-Heterocyclic Silylene (NHSi) is an uncharged heterocyclic chemical compound consisting of a divalent silicon atom bonded to two nitrogen atoms. The isolation of the first stable NHSi, also the first stable dicoordinate silicon compound, was reported in 1994 by Michael Denk and Robert West three years after Anthony Arduengo first isolated an N-heterocyclic carbene, the lighter congener of NHSis. Since their first isolation, NHSis have been synthesized and studied with both saturated and unsaturated central rings ranging in size from 4 to 6 atoms. The stability of NHSis, especially 6π aromatic unsaturated five-membered examples, make them useful systems to study the structure and reactivity of silylenes and low-valent main group elements in general. Though not used outside of academic settings, complexes containing NHSis are known to be competent catalysts for industrially important reactions. This article focuses on the properties and reactivity of five-membered NHSis.



















