
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.

Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure.

A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.

Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being copper(II) oxide or cupric oxide (CuO). Cuprous oxide is a red-coloured solid and is a component of some antifouling paints. The compound can appear either yellow or red, depending on the size of the particles. Copper(I) oxide is found as the reddish mineral cuprite.

Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow salt. It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. As a compound that is easy to isolate and purify, it is the principal source of cadmium for all commercial applications. Its vivid yellow color led to its adoption as a pigment for the yellow paint "cadmium yellow" in the 18th century.

Antimony trisulfide is found in nature as the crystalline mineral stibnite and the amorphous red mineral metastibnite. It is manufactured for use in safety matches, military ammunition, explosives and fireworks. It also is used in the production of ruby-colored glass and in plastics as a flame retardant. Historically the stibnite form was used as a grey pigment in paintings produced in the 16th century. In 1817, the dye and fabric chemist, John Mercer discovered the non-stoichiometric compound Antimony Orange, the first good orange pigment available for cotton fabric printing.

Cadmium telluride (CdTe) is a stable crystalline compound formed from cadmium and tellurium. It is mainly used as the semiconducting material in cadmium telluride photovoltaics and an infrared optical window. It is usually sandwiched with cadmium sulfide to form a p–n junction solar PV cell.

Cadmium selenide is an inorganic compound with the formula CdSe. It is a black to red-black solid that is classified as a II-VI semiconductor of the n-type. It is a pigment but applications are declining because of environmental concerns

Copper indium gallium (di)selenide (CIGS) is a I-III-VI2 semiconductor material composed of copper, indium, gallium, and selenium. The material is a solid solution of copper indium selenide (often abbreviated "CIS") and copper gallium selenide. It has a chemical formula of CuIn1−xGaxSe2, where the value of x can vary from 0 (pure copper indium selenide) to 1 (pure copper gallium selenide). CIGS is a tetrahedrally bonded semiconductor, with the chalcopyrite crystal structure, and a bandgap varying continuously with x from about 1.0 eV (for copper indium selenide) to about 1.7 eV (for copper gallium selenide).

Lorándite is a thallium arsenic sulfosalt with the chemical formula: TlAsS2. Though rare, it is the most common thallium-bearing mineral. Lorandite occurs in low-temperature hydrothermal associations and in gold and mercury ore deposits. Associated minerals include stibnite, realgar, orpiment, cinnabar, vrbaite, greigite, marcasite, pyrite, tetrahedrite, antimonian sphalerite, arsenic and barite.

Bismuth telluride is a gray powder that is a compound of bismuth and tellurium also known as bismuth(III) telluride. It is a semiconductor, which, when alloyed with antimony or selenium, is an efficient thermoelectric material for refrigeration or portable power generation. Bi2Te3 is a topological insulator, and thus exhibits thickness-dependent physical properties.

Cadmium oxide is an inorganic compound with the formula CdO. It is one of the main precursors to other cadmium compounds. It crystallizes in a cubic rocksalt lattice like sodium chloride, with octahedral cation and anion centers. It occurs naturally as the rare mineral monteponite. Cadmium oxide can be found as a colorless amorphous powder or as brown or red crystals. Cadmium oxide is an n-type semiconductor with a band gap of 2.18 eV at room temperature.
Tin(II) sulfide is a chemical compound of tin and sulfur. The chemical formula is SnS. Its natural occurrence concerns herzenbergite (α-SnS), a rare mineral. At elevated temperatures above 905 K, SnS undergoes a second order phase transition to β-SnS (space group: Cmcm, No. 63). In recent years, it has become evident that a new polymorph of SnS exists based upon the cubic crystal system, known as π-SnS (space group: P213, No. 198).

Thin-film solar cells are made by depositing one or more thin layers of photovoltaic material onto a substrate, such as glass, plastic or metal. Thin-film solar cells are typically a few nanometers (nm) to a few microns (µm) thick–much thinner than the wafers used in conventional crystalline silicon (c-Si) based solar cells, which can be up to 200 µm thick. Thin-film solar cells are commercially used in several technologies, including cadmium telluride (CdTe), copper indium gallium diselenide (CIGS), and amorphous thin-film silicon.

A copper indium gallium selenide solar cell is a thin-film solar cell used to convert sunlight into electric power. It is manufactured by depositing a thin layer of copper indium gallium selenide solid solution on glass or plastic backing, along with electrodes on the front and back to collect current. Because the material has a high absorption coefficient and strongly absorbs sunlight, a much thinner film is required than of other semiconductor materials.

Crystalline silicon or (c-Si) Is the crystalline forms of silicon, either polycrystalline silicon, or monocrystalline silicon. Crystalline silicon is the dominant semiconducting material used in photovoltaic technology for the production of solar cells. These cells are assembled into solar panels as part of a photovoltaic system to generate solar power from sunlight.
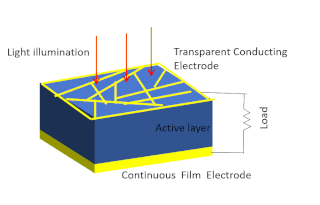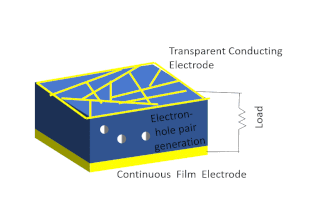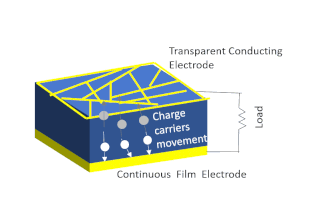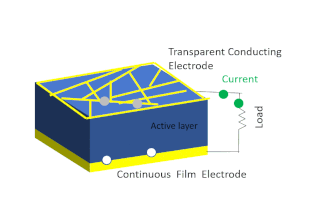
Solar-cell efficiency refers to the portion of energy in the form of sunlight that can be converted via photovoltaics into electricity by the solar cell.
Copper zinc tin sulfide (CZTS) is a quaternary semiconducting compound which has received increasing interest since the late 2000s for applications in thin film solar cells. The class of related materials includes other I2-II-IV-VI4 such as copper zinc tin selenide (CZTSe) and the sulfur-selenium alloy CZTSSe. CZTS offers favorable optical and electronic properties similar to CIGS (copper indium gallium selenide), making it well suited for use as a thin-film solar cell absorber layer, but unlike CIGS (or other thin films such as CdTe), CZTS is composed of only abundant and non-toxic elements. Concerns with the price and availability of indium in CIGS and tellurium in CdTe, as well as toxicity of cadmium have been a large motivator to search for alternative thin film solar cell materials. The power conversion efficiency of CZTS is still considerably lower than CIGS and CdTe, with laboratory cell records of 11.0 % for CZTS and 12.6 % for CZTSSe as of 2019.
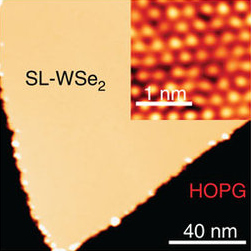
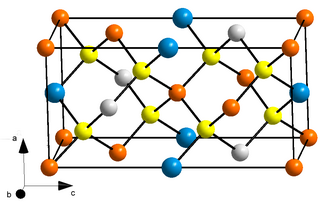
Tungsten diselenide is an inorganic compound with the formula WSe2. The compound adopts a hexagonal crystalline structure similar to molybdenum disulfide. The tungsten atoms are covalently bonded to six selenium ligands in a trigonal prismatic coordination sphere while each selenium is bonded to three tungsten atoms in a pyramidal geometry. The tungsten–selenium bond has a length of 0.2526 nm, and the distance between selenium atoms is 0.334 nm. It is a well studied example of a layered material. The layers stack together via van der Waals interactions. WSe2 is a very stable semiconductor in the group-VI transition metal dichalcogenides.


















