
High-temperature superconductors are defined as materials with critical temperature above 77 K, the boiling point of liquid nitrogen. They are only "high-temperature" relative to previously known superconductors, which function at even colder temperatures, close to absolute zero. The "high temperatures" are still far below ambient, and therefore require cooling. The first breakthrough of high-temperature superconductor was discovered in 1986 by IBM researchers Georg Bednorz and K. Alex Müller. Although the critical temperature is around 35.1 K, this new type of superconductor was readily modified by Ching-Wu Chu to make the first high-temperature superconductor with critical temperature 93 K. Bednorz and Müller were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-Tc materials are type-II superconductors.

Molybdenum disulfide is an inorganic compound composed of molybdenum and sulfur. Its chemical formula is MoS
2.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.

Tungsten disulfide is an inorganic chemical compound composed of tungsten and sulfur with the chemical formula WS2. This compound is part of the group of materials called the transition metal dichalcogenides. It occurs naturally as the rare mineral tungstenite. This material is a component of certain catalysts used for hydrodesulfurization and hydrodenitrification.

Tantalum(IV) sulfide is an inorganic compound with the formula TaS2. It is a layered compound with three-coordinate sulfide centres and trigonal prismatic or octahedral metal centres. It is structurally similar to molybdenum disulfide MoS2, and numerous other transition metal dichalcogenides. Tantalum disulfide has three polymorphs 1T-TaS2, 2H-TaS2, and 3R-TaS2, representing trigonal, hexagonal, and rhombohedral respectively.

Niobium monoxide is the inorganic compound with the formula NbO. It is a grey solid with metallic conductivity.
Iron(II) selenide refers to a number of inorganic compounds of ferrous iron and selenide (Se2−). The phase diagram of the system Fe–Se reveals the existence of several non-stoichiometric phases between ~49 at. % Se and ~53 at. % Fe, and temperatures up to ~450 °C. The low temperature stable phases are the tetragonal PbO-structure (P4/nmm) β-Fe1−xSe and α-Fe7Se8. The high temperature phase is the hexagonal, NiAs structure (P63/mmc) δ-Fe1−xSe. Iron(II) selenide occurs naturally as the NiAs-structure mineral achavalite.
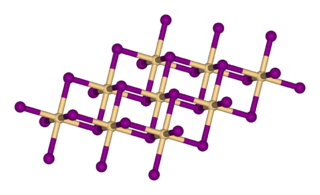
Titanium diselenide (TiSe2) also known as titanium(IV) selenide, is an inorganic compound of titanium and selenium. In this material selenium is viewed as selenide (Se2−) which requires that titanium exists as Ti4+. Titanium diselenide is a member of metal dichalcogenides, compounds that consist of a metal and an element of the chalcogen column within the periodic table. Many exhibit properties of potential value in battery technology, such as intercalation and electrical conductivity, although most applications focus on the less toxic and lighter disulfides, e.g. TiS2.
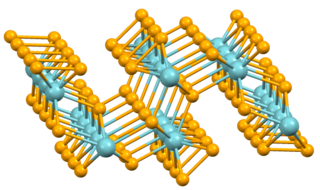
Niobium triselenide is an inorganic compound belonging to the class of transition metal trichalcogenides. It has the formula NbSe3. It was the first reported example of one-dimensional compound to exhibit the phenomenon of sliding charge density waves. Due to its many studies and exhibited phenomena in quantum mechanics, niobium triselenide has become the model system for quasi-1-D charge density waves.

Molybdenum diselenide is an inorganic compound of molybdenum and selenium. Its structure is similar to that of MoS
2. Compounds of this category are known as transition metal dichalcogenides, abbreviated TMDCs. These compounds, as the name suggests, are made up of a transition metals and elements of group 16 on the periodic table of the elements. Compared to MoS
2, MoSe
2 exhibits higher electrical conductivity.
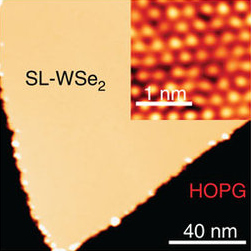
Tungsten diselenide is an inorganic compound with the formula WSe2. The compound adopts a hexagonal crystalline structure similar to molybdenum disulfide. The tungsten atoms are covalently bonded to six selenium ligands in a trigonal prismatic coordination sphere while each selenium is bonded to three tungsten atoms in a pyramidal geometry. The tungsten–selenium bond has a length of 0.2526 nm, and the distance between selenium atoms is 0.334 nm. It is a well studied example of a layered material. The layers stack together via van der Waals interactions. WSe2 is a very stable semiconductor in the group-VI transition metal dichalcogenides.

Transition-metal dichalcogenide (TMD or TMDC) monolayers are atomically thin semiconductors of the type MX2, with M a transition-metal atom (Mo, W, etc.) and X a chalcogen atom (S, Se, or Te). One layer of M atoms is sandwiched between two layers of X atoms. They are part of the large family of so-called 2D materials, named so to emphasize their extraordinary thinness. For example, a MoS2 monolayer is only 6.5 Å thick. The key feature of these materials is the interaction of large atoms in the 2D structure as compared with first-row transition-metal dichalcogenides, e.g., WTe2 exhibits anomalous giant magnetoresistance and superconductivity.
In materials science, the term single-layer materials or 2D materials refers to crystalline solids consisting of a single layer of atoms. These materials are promising for some applications but remain the focus of research. Single-layer materials derived from single elements generally carry the -ene suffix in their names, e.g. graphene. Single-layer materials that are compounds of two or more elements have -ane or -ide suffixes. 2D materials can generally be categorized as either 2D allotropes of various elements or as compounds.
A two-dimensional semiconductor is a type of natural semiconductor with thicknesses on the atomic scale. Geim and Novoselov et al. initiated the field in 2004 when they reported a new semiconducting material graphene, a flat monolayer of carbon atoms arranged in a 2D honeycomb lattice. A 2D monolayer semiconductor is significant because it exhibits stronger piezoelectric coupling than traditionally employed bulk forms. This coupling could enable applications. One research focus is on designing nanoelectronic components by the use of graphene as electrical conductor, hexagonal boron nitride as electrical insulator, and a transition metal dichalcogenide as semiconductor.

Molybdenum(IV) telluride, molybdenum ditelluride or just molybdenum telluride is a compound of molybdenum and tellurium with formula MoTe2, corresponding to a mass percentage of 27.32% molybdenum and 72.68% tellurium.
Platinum diselenide is a transition metal dichalcogenide with the formula PtSe2. It is a layered substance that can be split into layers down to three atoms thick. PtSe2 can behave as a metalloid or as a semiconductor depending on the thickness.
Verbeekite is a rare mineral consisting of palladium diselenide PdSe2. This transition metal dichalcogenide has an unusual monoclinic structure, with pairs of selenium atoms existing as dimers forming layers between palladium atom sheets. Unit cell dimensions are: a = 6.710, b = 4.154, c = 8.914 Å, β = 92.42 °, V = 248.24 Å3. Palladium diselenide has five polymorphs. Verbeekite can be synthesised at 11.5 GPa pressure and 1300 °C.

Rhenium diselenide is an inorganic compound with the formula ReSe2. It has a layered structure where atoms are strongly bonded within each layer. The layers are held together by weak Van der Waals bonds, and can be easily peeled off from the bulk material.

Tantalum diselenide is a compound made with tantalum and selenium atoms, with chemical formula TaSe2, which belongs to the family of transition metal dichalcogenides. In contrast to molybdenum disulfide (MoS2) or rhenium disulfide (ReS2), tantalum diselenide does not occur spontaneously in nature, but it can be synthesized. Depending on the growth parameters, different types of crystal structures can be stabilized.
Ferecrystals (FCs) are a class of layered materials consisting of atomically thin layers of a transition metal dichalcogenides (TMDC) stacked alternately with metal monochalcogenide layers.














