
A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride or vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.

Nickelocene is the organonickel compound with the formula Ni(η5-C5H5)2. Also known as bis(cyclopentadienyl)nickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it does not yet have any known practical applications.

Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.
Vanadocene dichloride is an organometallic complex with formula (η5-C5H5)2VCl2 (commonly abbreviated as Cp2VCl2). It is a structural analogue of titanocene dichloride but with vanadium(IV) instead of titanium(IV). This compound has one unpaired electron, hence Cp2VCl2 is paramagnetic. Vanadocene dichloride is a suitable precursor for variety of bis(cyclopentadienyl)vanadium(IV) compounds.

Chromocene is the organochromium compound with the formula [Cr(C5H5)2]. Like structurally related metallocenes, chromocene readily sublimes in a vacuum and is soluble in non-polar organic solvents. It is more formally known as bis(η5-cyclopentadienyl)chromium(II).

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives. Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

Organozirconium chemistry is the science of exploring the properties, structure, and reactivity of organozirconium compounds, which are organometallic compounds containing chemical bonds between carbon and zirconium. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.

Cyclopentadienylindium(I), C5H5In, is an organoindium compound containing indium in the +1 oxidation state. Commonly abbreviated to CpIn, it is a cyclopentadienyl complex with a half-sandwich structure. It was the first (1957) low-valent organoindium compound reported.
Zirconocene dichloride is an organozirconium compound composed of a zirconium central atom, with two cyclopentadienyl and two chloro ligands. It is a colourless diamagnetic solid that is somewhat stable in air.

Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.
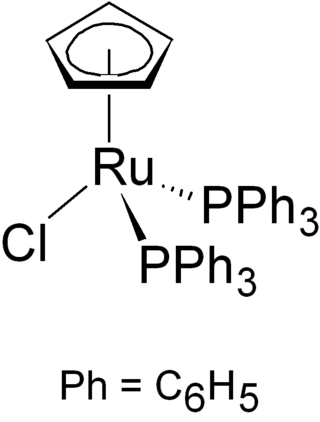
Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium is the organoruthenium half-sandwich compound with formula RuCl(PPh3)2(C5H5). It as an air-stable orange crystalline solid that is used in a variety of organometallic synthetic and catalytic transformations. The compound has idealized Cs symmetry. It is soluble in chloroform, dichloromethane, and acetone.
Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon (C) to vanadium (V) chemical bond. Organovanadium compounds find only minor use as reagents in organic synthesis but are significant for polymer chemistry as catalysts.
In organometallic chemistry, bent metallocenes are a subset of metallocenes. In bent metallocenes, the ring systems coordinated to the metal are not parallel, but are tilted at an angle. A common example of a bent metallocene is Cp2TiCl2. Several reagents and much research is based on bent metallocenes.
Molybdocene dichloride is the organomolybdenum compound with the formula (η5-C5H5)2MoCl2 and IUPAC name dichlorobis(η5-cyclopentadienyl)molybdenum(IV), and is commonly abbreviated as Cp2MoCl2. It is a brownish-green air- and moisture-sensitive powder. In the research laboratory, it is used to prepare many derivatives.

Titanocene pentasulfide is the organotitanium compound with the formula (C5H5)2TiS5, commonly abbreviated as Cp2TiS5. This metallocene exists as a bright red solid that is soluble in organic solvents. It is of academic interest as a precursor to unusual allotropes of elemental sulfur as well as some related inorganic rings.
Organoniobium chemistry is the chemistry of compounds containing niobium-carbon (Nb-C) bonds. Compared to the other group 5 transition metal organometallics, the chemistry of organoniobium compounds most closely resembles that of organotantalum compounds. Organoniobium compounds of oxidation states +5, +4, +3, +2, +1, 0, -1, and -3 have been prepared, with the +5 oxidation state being the most common.
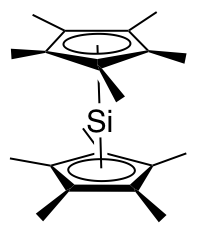
Decamethylsilicocene, (C5Me5)2Si, is a group 14 sandwich compound. It is an example of a main-group cyclopentadienyl complex; these molecules are related to metallocenes but contain p-block elements as the central atom. It is a colorless, air sensitive solid that sublimes under vacuum.

Molybdocene dihydride is the organomolybdenum compound with the formula (η5-C5H5)2MoH2. Commonly abbreviated as Cp2MoH2, it is a yellow air-sensitive solid that dissolves in some organic solvents.

Hafnocene dichloride is the organohafnium compound with the formula (C5H5)2HfCl2. It is a white solid that is sparingly soluble in some organic solvents. The lighter homologues zirconacene dichloride and titanocene dichloride have received much more attention. While hafnocene is only of academic interest, some more soluble derivatives are precatalysts for olefin polymerization. Moreso than the Zr analogue, this compound is highly resistant to reduction.

Tantalocene trihydride, or bis(η5-cyclopentadienyl)trihydridotantalum, is an organotanalum compound in the family of bent metallocenes consisting of two cyclopentadienyl rings and three hydrides coordinated to a tantalum center. Its formula is TaCp2H3, and it is a white crystalline compound that is sensitive to air. It is the first example of a molecular trihydride of a transition metal.















