A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride or vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.
Cyclopentadiene is an organic compound with the formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an unsaturated coordination sphere. These special anions are essential components of homogeneous alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the 14 valence electron cations [(C5H5)2ZrR]+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of alkenes. The popularization of non-coordinating anions has contributed to increased understanding of agostic complexes wherein hydrocarbons and hydrogen serve as ligands. Non-coordinating anions are important components of many superacids, which result from the combination of Brønsted acids and Lewis acids.

Nickelocene is the organonickel compound with the formula Ni(η5-C5H5)2. Also known as bis(cyclopentadienyl)nickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it does not yet have any known practical applications.

In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.

Chromocene is the organochromium compound with the formula [Cr(C5H5)2]. Like structurally related metallocenes, chromocene readily sublimes in a vacuum and is soluble in non-polar organic solvents. It is more formally known as bis(η5-cyclopentadienyl)chromium(II).

Tris(pentafluorophenyl)borane, sometimes referred to as "BCF", is the chemical compound (C6F5)3B. It is a white, volatile solid. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the BC3 core is planar. It has been described as the “ideal Lewis acid” because of its high thermal stability and the relative inertness of the B-C bonds. Related fluoro-substituted boron compounds, such as those containing B−CF3 groups, decompose with formation of B-F bonds. Tris(pentafluorophenyl)borane is thermally stable at temperatures well over 200 °C, resistant to oxygen, and water-tolerant.

Decamethyldizincocene is an organozinc compound with the formula [Zn2(η5–C5Me5)2]. It is the first and an unusual example of a compound with a Zn-Zn bond. Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the presence of oxygen and reacts with water. It is stable at room temperature and especially soluble in diethyl ether, benzene, pentane, or tetrahydrofuran.
In organometallic chemistry, a transition metal indenyl complex is a coordination compound that contains one or more indenyl ligands. The indenyl ligand is formally the anion derived from deprotonation of indene. The η5-indenyl ligand is related to the η5cyclopentadienyl anion (Cp), thus indenyl analogues of many cyclopentadienyl complexes are known. Indenyl ligands lack the 5-fold symmetry of Cp, so they exhibit more complicated geometries. Furthermore, some indenyl complexes also exist with only η3-bonding mode. The η5- and η3-bonding modes sometimes interconvert.

Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
Boron monofluoride or fluoroborylene is a chemical compound with the formula BF, one atom of boron and one of fluorine. It is an unstable gas, but it is a stable ligand on transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. It can also be called a borylene, as it contains boron with two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons.
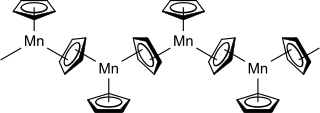
Manganocene or bis(cyclopentadienyl)manganese(II) is an organomanganese compound with the formula [Mn(C5H5)2]n. It is a thermochromic solid that degrades rapidly in air. Although the compound is of little utility, it is often discussed as an example of a metallocene with ionic character.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.

Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula [(η5-C5H5)Fe(CO)2]2, often abbreviated to Cp2Fe2(CO)4, [CpFe(CO)2]2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).
Magnesocene, also known as bis(cyclopentadienyl)magnesium(II) and sometimes abbreviated as MgCp2, is an organometallic compound with the formula Mg(η5-C5H5)2. It is an example of an s-block main group sandwich compound, structurally related to the d-block element metallocenes, and consists of a central magnesium atom sandwiched between two cyclopentadienyl rings.

An N-Heterocyclic silylene (NHSi) is an uncharged heterocyclic chemical compound consisting of a divalent silicon atom bonded to two nitrogen atoms. The isolation of the first stable NHSi, also the first stable dicoordinate silicon compound, was reported in 1994 by Michael Denk and Robert West three years after Anthony Arduengo first isolated an N-heterocyclic carbene, the lighter congener of NHSis. Since their first isolation, NHSis have been synthesized and studied with both saturated and unsaturated central rings ranging in size from 4 to 6 atoms. The stability of NHSis, especially 6π aromatic unsaturated five-membered examples, make them useful systems to study the structure and reactivity of silylenes and low-valent main group elements in general. Though not used outside of academic settings, complexes containing NHSis are known to be competent catalysts for industrially important reactions. This article focuses on the properties and reactivity of five-membered NHSis.

A lanthanocene is a type of metallocene compound that contains an element from the lanthanide series. The most common lanthanocene complexes contain two cyclopentadienyl anions and an X type ligand, usually hydride or alkyl ligand.
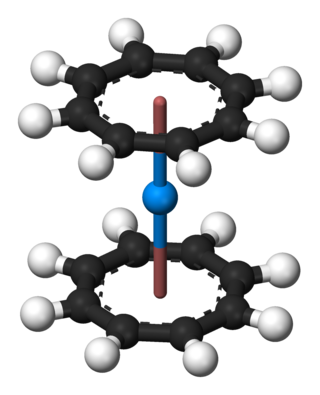
Organothorium chemistry describes the synthesis and properties of organothorium compounds, chemical compounds containing a carbon to thorium chemical bond.