 | |
| Names | |
|---|---|
| IUPAC name Triethylindium | |
| Other names Indium triethyl, triethylindigane, indiumtriethyl, TEI, TEIn | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.011.905 |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
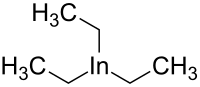
| In(CH2CH3)3 | |
| Molar mass | 202.004 g·mol−1 |
| Appearance | Colorless liquid [1] |
| Density | 1.384 g/cm3 [1] |
| Melting point | −32 °C (−26 °F; 241 K) [1] |
| Boiling point | 144 °C (291 °F; 417 K) |
| Reacts violently [1] | |
Refractive index (nD) | 1.5380 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Causes severe skin burns and serious eye damage. Spontaneously ignites on air. |
| GHS labelling: | |
  | |
| Danger | |
| H250, H314 | |
| P210, P222, P231, P233, P260, P264, P280, P301+P330+P331, P302+P335+P334, P302+P361+P354, P304+P340, P305+P354+P338, P316, P321, P363, P370+P378, P405, P501 | |
| Related compounds | |
Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Triethylindium is an organometallic compound. Its chemical formula is In(CH2CH3)3. [2] [3]